Abstract
Congenital nephrotic syndrome (CNS) was primarily considered one disease entity. Hence, one treatment protocol was proposed in the beginning to all CNS patients. Today, with the help of gene diagnostics, we know that CNS is a heterogeneous group of disorders and therefore, different treatment protocols are needed. The most important gene defects causing CNS are NPHS1, NPHS2, WT1, LAMB2, and PLCE1. Before active treatment, all infants with CNS died. It was stated already in the mid-1980s that intensive medical therapy followed by kidney transplantation (KTx) should be the choice of treatment for infants with severe CNS. In Finland, early aggressive treatment protocol was adopted from the USA and further developed for treatment of children with the Finnish type of CNS. The aim of this review is to state reasons for “early aggressive treatment” including daily albumin infusions, intensified nutrition, and timely bilateral nephrectomy followed by KTx at the age of 1–2 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital nephrotic syndrome (CNS) manifests within the first 3 months of age, and is differentiated from infantile nephrotic syndrome, which appears later during the first 1–2 years of life and mostly has a more favorable prognosis [1]. CNS of the Finnish type (CNF; NPHS1, MIM#256300F) was clinically described by Niilo Hallman in 1956 [2] and Reijo Norio reported its autosomal recessive inheritance 10 years later [3]. CNF is defined as heavy proteinuria, severe hypoproteinemia, edema, and secondary manifestations due to heavy proteinuria detected shortly after birth. Familial and sporadic CNS cases were described in infants without Finnish background already in the 1940s and 1950s, suggesting that primary CNS was not a single entity [4]. In 1998, Marjo Kestilä and coworkers identified mutations in the NPHS1 gene and named the product of the gene as nephrin [5]. Around 90% of the Finnish CNF patients have two truncating NPHS1 mutations (Fin-major/Fin-major 60%, Fin-major/Fin-minor 20%, Fin-minor/Fin-minor 10%) [5]. Both mutations lead to a total absence of nephrin molecules in the podocyte slit diaphragm and severe damage of this structure [6]. Worldwide, more than 200 NPHS1 mutations with variable clinical severity have been identified [7,8,9,10,11,12,13].
Other important genetic defects affecting the glomerular filtration barrier are found in NPHS2 (podocin), WT1 (Wilms tumor protein 1), LAMB2 (laminin beta 2), and PLCE1 (phospholipase C epsilon 1) [14,15,16,17]. As is the case with NPHS1, these gene defects cause mostly isolated CNS and, less often, syndromic disorders. The magnitude of protein losses and development of kidney failure are, however, variable, and the severity of the disease may vary even between patients having the same mutation. Clinical features of severe CNS are failure to thrive, difficulties in controlling hydration status, and complications, like thrombosis or repeated sepsis.
CNF has been considered a prototype of CNS and its management is still challenging [18,19,20]. Due to the variable severity of the disease, CNS patients have been treated during the past 2–3 decades according to variable protocols including an early bilateral nephrectomy and kidney transplantation (KTx), unilateral nephrectomy in an attempt to delay dialysis and transplantation, and conservative treatment until development of kidney failure [21]. Due to the rarity of CNS and its genetic and clinical variability, there is not enough evidence to define one treatment strategy for all CNS patients. The aim of this review is to state reasons when and why to use “early aggressive treatment,” including intensified nutrition, albumin infusions, timely bilateral nephrectomy, dialysis, and KTx at the age of 1–2 years.
Evolution of treatment for severe CNS
The outcome of CNS infants was originally dismal and was related to heavy urinary protein losses. Niilo Huttunen published data on 75 Finnish CNF patients followed over the years 1965–1973 [22]. The children had nutritional support but no one received albumin infusions. Edema and/or abdominal distension was noticed in children before 2 months of age. Over half of the children died before 6 months of age, with mean patient survival of 7.6 months. The oldest child survived 2.2 years. Infection was the most common cause of death (31%), 19% died due to thrombotic complications, and in 43%, the cause of death remained unknown (mostly hemodynamic collapse). None of the children developed uremia before their death.
Based on three CNS children transplanted successfully in Minnesota in the early 1970s, KTx was proposed as the only way to provide a permanent cure and good quality of life for these children [23]. Hoyer et al. proposed further that KTx should be performed before kidney failure to decrease early mortality and growth failure [23]. Active treatment, which included high caloric, high protein, and low sodium diet, was offered in Minnesota to infants since 1971 [4]. The infants also received diuretics and IV albumin to allow sufficient fluid intake for caloric needs and to minimize edema. Despite active treatment, 25% of the patients died before they were transplanted, 85% suffered from bacterial infections, all had growth retardation, and 93% showed developmental delay or neurological abnormalities before KTx. Most of the patients underwent bilateral nephrectomy prior to the transplantation and they became dramatically less irritable, more active, and their appetite improved [4]. Mahan et al. concluded that CNS should no longer be considered lethal and that intensive medical therapy followed by KTx should be the treatment of choice [4].
Intensified treatment protocol for CNF children
The active Finnish treatment strategy for CNF infants was adopted from Minnesota to prevent early death and complications [1]. A three-stage protocol was regarded feasible in treatment of infants with severe CNS: (1) Management of nephrosis from birth to the age of 6–10 months (weight 7 kg), (2) bilateral nephrectomy and peritoneal dialysis (PD) for 3–6 months, (3) KTx with extra peritoneal engraftment when the weight of 10 kg has been reached (usually 1–1.5 years of age).
Due to high frequency of NPHS1-associated CNS in our country, intensified treatment is started for all 0–3-month-old infants with severe hypoalbuminemia, heavy proteinuria (after albumin substitution), and clinical signs of nephrosis. The therapy is individually adjusted based on the clinical response and genetic analysis. Especially in infants with a “mild” genotype (missense mutation in one or two of the NPHS1 alleles, or other mutated genes) and stable clinical status, weaning off the parenteral therapy should be tried after the first few weeks. This, however, rarely succeeds in our patient material.
So far, 130 CNF children have been treated with this protocol. Two (1.5%) of the infants died during the nephrotic stage, seven on dialysis (5.4%), and seven (5.4%) after KTx. In total, close to 90% of the children have survived and managed quite well with their kidney grafts.
Management of nephrosis
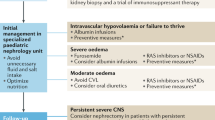
During the first month, the treatment of infants with CNS strives for controlling edema, preventing and treating complications like infections and thromboses, and providing optimal nutrition to allow best possible growth and development (Table 1) [1, 18]. Diet is energy-rich (130 kcal/kg/day) and protein-rich (3–4 g/kg/day). Albumin infusions (1–4 g/kg/day) combined with intravenous furosemide (1–3 mg/kg/day) are initially divided into three 2-h infusions and after a few weeks given as one nightly infusion or two shorter infusions. The goal of the albumin/furosemide infusions is to minimize edema and hemodynamic problems. The dosing is based on weight and other clinical signs—not on plasma albumin levels, which remain low. In case of increased edema, which is common especially during infections, the nightly albumin/furosemide infusions are divided into 4 daily doses to increase diuresis.
In practice, parenteral infusions require central vein catheters, which are usually placed at the age of a few weeks. They carry a risk for thrombotic events, especially since children with CNF show hypercoagulopathy, caused by urinary loss of coagulation factors. Intravenous ATIII (50 units/kg) is given before all invasive procedures and sodium warfarin is used in all infants with INR target level of 2–2.5 [18]. We have observed clearly fewer thrombotic complications since systematic use of anticoagulation. In the study of the European Society for Pediatric Nephrology Dialysis Working Group, the incidence of thrombosis in 71 CNS patients treated between 2010 and 2015 was 13% [20]. However, no statistical difference was found between patients on antithrombotic prophylaxis (67% received heparin and 33% warfarin sodium) and those not on prophylaxis. In our experience, immunoglobulin infusions and antibiotic prophylaxis are not sufficient in prevention of infections and are therefore not used routinely [18, 24].
Reduction of protein excretion by ACE inhibitors (and indomethacin) has been achieved in CNS infants with genetic defects [20, 25]. This “antiproteinuric therapy” is worthwhile trying especially in cases with milder protein excretion and/or a missense mutation (amino acid change) in one or both NPHS1 alleles, or other nephrosis genes. Among our patients, an infant with Fin-major/R743C genotype responded permanently to ACE inhibitor therapy [26]. On the other hand, patients with truncating mutations have never done that. In CNF patients, even “mild” missense mutations may lead to defective trafficking of encoded protein and its absence on the podocyte surface, which resembles the situation with truncating mutations [27].
Dialysis
Dialysis may be needed for the treatment of CNS children either after the development of kidney failure or after bilateral nephrectomy.
Continuous PD was introduced to infants already in the 1980s [28, 29]. Infections and poor growth were major problems during the early years [30,31,32]. Subsequently, the results improved, and it became evident that the most important risk factor for mortality in infants is their non-renal comorbidity [33, 34]. Laakkonen et al. prospectively studied 21 Finnish infants starting PD at mean age 0.59 years [35]. CNF was the primary diagnosis in 71% and bilateral nephrectomy was performed in all of them. Catch-up growth was documented in 57%, and 29% had neurological abnormalities since birth which did not progress during PD [35].
During the past years, the use of hemodialysis (HD) has increased in infants and toddlers with kidney failure. HD is feasible also in nephrectomized CNS infants. In particular, patients who will undergo a living-related donor transplantation and live relatively close to the hospital may be treated for a few weeks with HD prior to KTx. HD is a valid treatment also for infants with problems in the PD therapy (infections and technical problems).
Kidney transplantation
A KTx program for infants was started in Finland after encouraging a report by Mahan et al. [1]. Holmberg et al., in the mid-1990s, reported excellent 3-year results for 32 CNF patients transplanted after bilateral nephrectomy and treated with PD for at least 3 months [1]. Initially, the results of KTx in children less than 2–5 years of age were less impressive than in older recipients, but patient outcome and graft survival improved clearly over the next decades. Laine et al. in 1994 reported comparable results for KTx in children under 5 years of age and older ones [36]. Five years later, Qvist et al. reported excellent 7-year KTx outcome for children under 5 years of age, of which 84% had CNF as the primary diagnosis [37]. Patient survival was 100% and graft survival was equal for children under and over 2 years of age. Two later studies were in accordance with that of Qvist et al., stating that the age does not affect 10-year graft survival [38, 39]. Today, young children have longer half-life of their kidney graft than older children [38, 39].
In most of the children with severe CNS, it is clear that quality of life is better and risk for complications lower after KTx compared with the nephrotic or uremic/dialysis periods. KTx has been performed on young infants with a weight of < 5 kg and adult-sized grafts have been successfully transplanted into infants weighing 7–10 kg [40]. In our experience, KTx with extra peritoneal engraftment can be safely performed for a 10-kg infant.
Thrombotic events used to be a major problem when transplanting small children. Normalization of the coagulation factor levels before KTx (during the dialysis stage), proper placement of the graft (proximal vessel anastomoses), avoidance of compression and circulatory problems by postponing the closure of fascia for a few days after KTx when necessary, and abundant fluid therapy peri- and post-operatively are essential in avoiding thrombotic events. Using this protocol, we have lost one graft (0.7%) due to thrombosis in CNS children, which is less than that reported in 1–12-year-aged children (around 2%) [40].
Another important issue in infant KTx is the risk for Epstein–Barr virus (EBV) infections and post-transplantation lymphoproliferative disease (PTLD) after KTx. A small child who is seronegative for EBV at the time of KTx has increased risk for PTLD. In our center, PTLD was diagnosed in four school-aged children transplanted as infants due to CNS (4%) as compared with three non-CNS children (2%) transplanted after 2 years of age (total 252 children) [40].
Long-term outcome
During the nephrotic stage, psychomotor delay caused by muscle weakness is common in children with severe CNS [4]. Typically, the children show dramatic improvement in their developmental skills within 1 year after KTx [4]. Also, the cardiac hypertrophy often seen in the nephrotic stage reverses.
Avoidance of neurological complications is crucial in the management of CNS children. Impairment in intellectual performance and motor skills among KTx children has been mainly associated with neurological comorbidity, hypertensive crises, and seizures during dialysis, as well as lower socio-economic status, but they have been observed also in absence of any comorbidities [41, 42]. Haavisto et al. studied 50 Finnish children 6.9 years after their first KTx performed over the years 1993–2008 at mean age of 3.7 years (44% had NPHS1 as their primary disease) with respect to cognitive difficulties [43]. Children with KTx scored lower in neuropsychological assessment, both for the verbal and visuospatial domains, compared with the controls. Better cognitive outcome was associated with absence of neurological comorbidity, but also with younger age, shorter disease duration, and better long-term kidney function [43]. Even though CNF children are mostly born with a lower gestational age and birth weight, and are transplanted earlier than children with other diagnoses, they scored higher on all measures than children with other diagnoses. Haavisto et al. concluded that children with KTx later in their life experience longer disease duration, which places them at risk for more neurodevelopmental deficits [43].
Infants receiving living or deceased donor grafts have estimated graft survival of at least 80% at 10 years [38]. Thus, many CNS patients transplanted at the age of 1–2 years need a new graft as young adults. The likelihood, however, is not greater compared with those transplanted 2–5 years later [38]. On the contrary, since children with severe CNS are prone to complications, later KTx might negatively impact their later life.
Conclusions
CNS is not one disease with a single-treatment protocol. The magnitude of urinary protein losses is important in deciding the treatment strategy. In case of massive proteinuria (100–150 g/L), severe hypoproteinemia may easily lead to edema, thrombotic complications, and sometimes hemodynamic compromise. On the other hand, an infant with proteinuria of 5–20 g/L and gradually developing uremia can manage without aggressive parenteral therapy. Whether to use bilateral, unilateral, or no nephrectomy should be based on severity of CNS and the experience in the treating center. They all might work, but bilateral nephrectomy terminates the harmful proteinuria, and active treatment during nephrosis helps children to grow and develop their motor skills. The quality of life is better after KTx than before it. Treatment results have improved over the last decades; therefore, the decision on timing nephrectomy should be made individually. Nephrectomy may be postponed in patients with severe CNS who do not develop any specific complications during the nephrotic period. Kidney transplantation results in infants are today excellent, and according to the latest studies, infants seem to have the best results, and the outcome of CNS infants does not differ from infants with other diagnoses. Hence, it seems justified to perform early KTx in infants with severe CNS.
Key summary points
-
1.
Not enough evidence is available to define optimal treatment strategy for CNS due to its rarity and large genetic variation in patient population.
-
2.
Early aggressive treatment for patients with severe CNS includes daily albumin infusions, intensified nutrition, and timely bilateral nephrectomy followed by early KTx.
-
3.
Psychomotor delay is related to muscle weakness and KTx improves developmental skills dramatically.
-
4.
Infants have nowadays the best long-term KTx outcome, and results of NPHS1 patients do not differ from those of infants with other diagnoses than CNS.
-
5.
Short period of protein deficiency during first year of life, combined with short dialysis and early KTx, seems to be less detrimental for neurological development in children with severe CNS; hence, early KTx should be considered.
-
6.
Studies are needed to compare long-term and neurocognitive outcome between children with severe CNS treated aggressively and conservatively.
References
Holmberg C, Antikainen M, Rönnholm K, Ala-Houhala M, Jalanko H (1995) Management of congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol 9:87–93
Hallman N, Hjelt L, Ahvenainen EK (1956) Nephrotic syndrome in newborns and young infants. Ann Paediatr Fenn 2:228–241
Norio R (1966) Heredity in the congenital nephrotic syndrome: a genetic study of 57 Finnish families with a review of reported cases. Ann Paediatr Fenn 12(Suppl 27):1–94
Mahan JD, Mauer SM, Sibley RK, Vernier RL (1984) Congenital nephrotic syndrome: evolution of medical management and results of renal transplantation. J Pediatr 105:549–557
Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan C, Peltonen L, Holmberg C, Olsen A, Tryggvason K (1998) Positionally cloned gene for a novel glomerula protein-nephrin-is mutated in congenital nephrotic syndrome. Mol Cell 1:575–582
Wartiovaara J, Ofverstedt L, Khoshnoodi J, Zhang J, Makela E, Sandin S, Ruotsalainen V, Cheng R, Jalanko H, Skoglund U, Tryggvason K (2004) Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest 114:1475–1483
Lenkkeri U, Mannikko M, McCready P, Lamerdin J, Gribouval O, Niaudet PM, Antignac CK, Kashtan CE, Homberg C, Olsen A, Kestila M, Tryggvason K (1999) Structure of the gene for congenital nephrotic syndrome of the finnish type (NPHS1) and characterization of mutations. Am J Hum Genet 64:51–61
Beltcheva O, Martin P, Lenkkeri U, Tryggvason K (2001) Mutation spectrum in the nephrin gene (NPHS1) in congenital nephrotic syndrome. Hum Mutat 17:368–373
Koziell A, Grech V, Hussain S, Lee G, Lenkkeri U, Tryggvason K, Scambler P (2002) Genotype/phenotype correlations of NPHS1 and NPHS2 mutations in nephrotic syndrome advocate a functional inter-relationship in glomerular filtration. Hum Mol Genet 11:379–388
Heeringa SF, Vlangos CN, Chernin G, Hinkes B, Gbadegesin R, Liu J, Hoskins BE, Ozaltin F, Hildebrandt F, Members of the APN Study Group (2008) Thirteen novel NPHS1 mutations in a large cohort of children with congenital nephrotic syndrome. Nephrol Dial Transplant 23:3527–3533
Godefroid N, Dahan K (2010) Expanding the clinical spectrum of congenital nephrotic syndrome caused by NPHS1 mutations. Nephrol Dial Transplant 25:2837–2839
Machuca E, Benoit G, Nevo F, Tete MJ, Gribouval O, Pawtowski A, Brandstrom P, Loirat C, Niaudet P, Gubler MC, Antignac C (2010) Genotype-phenotype correlations in non-Finnish congenital nephrotic syndrome. J Am Soc Nephrol 21:1209–1217
Schoeb DS, Chernin G, Heeringa SF, Matejas V, Held S, Vega-Warner V, Bockenhauer D, Vlangos CN, Moorani KN, Neuhaus TJ, Kari JA, MacDonald J, Saisawat P, Ashraf S, Ovunc B, Zenker M, Hildebrandt F, Gesselschaft fur Paediatrische Nephrologie Study Group (2010) Nineteen novel NPHS1 mutations in a worldwide cohort of patients with congenital nephrotic syndrome (CNS). Nephrol Dial Transplant 25:2970–2976
Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gruber M, Niaudet P, Antignac C (2000) NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24:349–354
Jeanpierre C, Denamur E, Henry I, Cabanis MO, Luce S, Cécille A, Elion J, Peuchmaur M, Loirat C, Niaudet P, Gubler MC, Junien C (1998) Identification of constitutional WT1 mutations, in patients with isolated diffuse mesangial sclerosis, and analysis of genotype/phenotype correlations by use of a computerized mutation database. Am J Hum Genet 62:824–833
Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A (2004) Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13:2625–2632
Gbadegesin R, Hinkes BG, Hoskins BE, Vlangos CN, Heeringa SF, Liu J, Loirat C, Ozaltin F, Hashmi S, Ulmer F, Cleper R, Ettenger R, Antignac C, Wiggins RC, Zenker M, Hildebrandt F (2008) Mutations in PLCE1 are a major cause of isolated diffuse mesangial sclerosis (IDMS). Nephrol Dial Transplant 23:1291–1297
Jalanko H (2009) Congenital nephrotic syndrome. Pediatr Nephrol 24:2121–2128
Hölttä T, Bonthuis M, Van Stralen KJ, Bjerre A, Topaloglu R, Ozaltin F, Holmberg C, Harambat J, Jager KJ, Schaefer F, Groothoff JW (2016) Timing of renal replacement therapy does not influence survival and growth in children with congenital nephrotic syndrome caused by mutations in NPHS1: data from the ESPN/ERA-EDTA Registry. Pediatr Nephrol 31:2317–2325
Dufek S, Holtta T, Trautmann A, Ylinen E, Alpay H, Ariceta G, Aufricht C, Bacchetta J, Bakkaloglu SA, Bayazit A, Cicek RY, Dursun I, Duzova A, Ekim M, Iancu D, Jankauskiene A, Klaus G, Paglialonga F, Pasini A, Printza N, Said Conti V, do Sameiro Faria M, Schmitt CP, Stefanidis CJ, Verrina E, Vidal E, Vondrak K, Webb H, Zampetoglou A, Bockenhauer D, Edefonti A, Shroff R (2019) Management of children with congenital nephrotic syndrome: challenging treatment paradigms. Nephrol Dial Transplant 34:1369–1377. https://doi.org/10.1093/ndt/gfy165
Kovacevic L, Reid CJ, Rigden SP (2003) Management of congenital nephrotic syndrome. Pediatr Nephrol 18:426–430
Huttunen N-P (1976) Congenital nephrotic syndrome of Finnish type. Study of 75 patietns. Arch Dis Child 51:344–348
Hoyer JR, Kjellstrand CM, Simmons RL, Naharian JS, Mauer SM, Buselmeier TJ, Michael AF, Vernier RL (1973) Successful renal transplantation in 3 children with congenital nephrotic syndrome. Lancet 1(7817):1410–1412
Ljungberg P, Holmberg C, Jalanko H (1997) Infections in infants with congenital nephrosis of the Finnish type. Pediatr Nephrol 11:148–152
Licht C, Eifinger F, Mostafa G, Offner G, Michalk DV, Querfeld U (2000) A stepwise approach to the treatment of early onset nephrotic syndrome. Pediatr Nephrol 14:1077–1082
Patrakka J, Kestilä M, Wartiovaara J, Ruotsalainen V, Tissari P, Lenkkeri U, Männikkö M, Visapää I, Holmberg C, Rapola J, Tryggvason K, Jalanko H (2000) Congenital nephrotic syndrome (NPHS1): features resulting from different mutations in Finnish patients. Kidney Int 58:972–980
Liu XL, Doné SC, Yan K, Kilpeläinen P, Pikkarainen T, Tryggvason K (2000) Defective trafficking of nephrin missense mutants rescued by a chemical chaperone. J Am Soc Nephrol 15:1731–1738
Oreopoulos DG, Katirtzoglou A, Arbus G, Cordy B (1979) Dialysis and transplantation in young children. Br Med J 1:1628–1629
Hölttä TM, Rönnholm KA, Jalanko H, Ala-Houhala M, Antikainen M, Holmberg C (1997) Peritoneal dialysis in children under 5 years of age. Perit Dial Int 17:573–580
Bunchman TE (1995) Chronic dialysis in the infant less than 1 year of age. Pediatr Nephrol 9:S18–S22
Howard RL, Millspaugh J, Teitelbaum I (1990) Adult and pediatric peritonitis rates in a home dialysis program: comparison of continuous ambulatory and continuous cycling peritoneal dialysis. Am J Kidney Dis 16:469–472
Warady BA, Hebert D, Sullivan EK, Alexander SR, Tejani A (1997) Renal transplantation, chronic dialysis, and chronic renal insufficiency in children and adolescents. The 1995 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 11:49–64
Lederman SE, Scanes ME, Fernando ON, Duffy PG, Madden SJ, Trompeter RS (2000) Long-term outcome of peritoneal dialysis in infants. J Pediatr 136:24–29
Wood EG, Hand M, Briscoe DM, Donaldson LA, Yiu V, Harley FL, Warady BA, Ellis EN, North American Pediatric Renal Transplant Cooperative Study (2001) Risk factors for mortality in infants and young children on dialysis. Am J Kidney Dis 37:573–579
Laakkonen H, Lönnqvist T, Valanne L, Karikoski R, Holmberg C, Rönnholm K (2011) Neurological development in 21 children on peritoneal dialysis in infancy. Pediatr Nephrol 26:1863–1871. https://doi.org/10.1007/s00467-011-1893-y
Laine J, Holmberg C, Salmela K, Jalanko H, Sairanen H, Peltola K, Rönnholm K, Eklund B, Wikström S, Leijala M (1994) Renal transplantation in children with emphasis on young patients. Pediatr Nephrol 8:313–319
Qvist E, Laine J, Rönnholm K, Jalanko H, Leijala M, Holmberg C (1999) Graft function 5-7 years after renal transplantation in early childhood. Transplantation 67:1043–1049
Andreoni KA, Forbes R, Andreoni RM, Phillips G, Stewart H, Ferris M (2013) Age-related kidney transplant outcomes: health disparities amplified in adolescence. JAMA Intern Med 173:1524–1532
Hölttä T, Gordin D, Rahkonen O, Turanlahti M, Holmström M, Tainio J, Rönnholm K, Jalanko H (2020) Good long-term renal graft survival and low incidence of cardiac pathology in adults after short dialysis period and renal transplantation in early childhood. Transpl Int 33:89–97. https://doi.org/10.1111/tri.13521
Jalanko H, Mattila I, Holmberg C (2016) Renal tranplantation in infants. Pediatr Nephrol 31:725–735
Fennell RS 3rd, Rasbury WC, Fennell EB, Morris MK (1984) Effects of kidney transplantation on cognitive performance in a pediatric population. Pediatrics 74:273–278
Falger J, Latal B, Landolt MA, Lehmann P, Neuhaus TJ, Laube GF (2008) Outcome after renal transplantation. Part I: intellectual and motor performance. Pediatr Nephrol 23:1339–1345. https://doi.org/10.1007/s00467-008-0795-0
Haavisto A, Korkman M, Holmberg C, Jalanko H, Qvist E (2012) Neuropsychological profile of children with kidney transplants. Nephrol Dial Transplant 27:2594–2601. https://doi.org/10.1093/ndt/gfr650
Funding
Open access funding provided by University of Helsinki including Helsinki University Central Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hölttä, T., Jalanko, H. Congenital nephrotic syndrome: is early aggressive treatment needed? Yes. Pediatr Nephrol 35, 1985–1990 (2020). https://doi.org/10.1007/s00467-020-04578-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04578-4