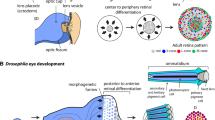
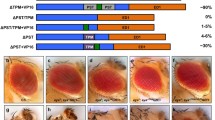
Abstract
Eyes absent (EYA) and Sine oculis (SO/SIX) proteins function as transcriptional activation complexes and play essential roles in organogenesis during embryonic development in regulating cell proliferation and survival and coordination of particular differentiation programs. Mutations of the Eya and So/Six genes cause profound developmental defects in organisms as diverse as flies, frogs, fish, mice, and humans. EYA proteins also possess an intrinsic phosphatase activity, which is essential for normal development. Here, we review crucial roles of EYA and SO/SIX in development and disease in mice and humans.




Similar content being viewed by others
References
Serikaku MA, O'Tousa JE (1994) sine oculis is a homeobox gene required for Drosophila visual system development. Genetics 138:1137–1150
Xu PX, Woo I, Her H, Beier DR, Maas RL (1997) Mouse Eya homologues of the Drosophila Eyes absent gene require Pax6 for expression in lens and nasal placode. Development 124:219–231
Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C (1997) A human homologue of the Drosophila Eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet 15:157–164
Bonini NM, Leiserson WM, Benzer S (1993) The Eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell 72:379–395
Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL (1994) The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12:977–996
Zimmerman JE, Bui QT, Steingrimsson E, Nagle DL, Fu W, Genin A, Spinner NB, Copeland NG, Jenkins NA, Bucan M, Bonini NM (1997) Cloning and characterization of two vertebrate homologs of the Drosophila Eyes absent gene. Genome Res 7:128–141
Sahly I, Andermann P, Petit C (1999) The zebrafish eya1 gene and its expression pattern during embryogenesis. Dev Genes Evol 209:399–410
Seo HC, Curtiss J, Mlodzik M, Fjose A (1999) Six class homeobox genes in Drosophila belong to three distinct families and are involved in head development. Mech Dev 83:127–139
Kawakami K, Sato S, Ozaki H, Ikeda K (2000) Six family genes–structure and function as transcription factors and their roles in development. Bioessays 22:616–626
Dozier C, Kagoshima H, Niklaus G, Cassata G, Burglin TR (2001) The Caenorhabditis elegans Six/sine oculis class homeobox gene ceh-32 is required for head morphogenesis. Dev Biol 236:289–303
Hoshiyama D, Iwabe N, Miyata T (2007) Evolution of the gene families forming the Pax/Six regulatory network: isolation of genes from primitive animals and molecular phylogenetic analyses. FEBS Lett 581:1639–1643
Pineda D, Gonzalez J, Callaerts P, Ikeo K, Gehring WJ, Salo E (2000) Searching for the prototypic eye genetic network: sine oculis is essential for eye regeneration in planarians. Proc Natl Acad Sci USA 97:4525–4529
Bessarab DA, Chong SW, Korzh V (2004) Expression of zebrafish six1 during sensory organ development and myogenesis. Dev Dyn 230:781–786
Takeda Y, Hatano S, Sentoku N, Matsuoka M (1999) Homologs of animal Eyes absent (eya) genes are found in higher plants. Mol Gen Genet 262:131–138
Bebenek IG, Gates RD, Morris J, Hartenstein V, Jacobs DK (2004) sine oculis in basal Metazoa. Dev Genes Evol 214:342–351
Treisman JE (1999) A conserved blueprint for the eye? Bioessays 21:843–850
Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D (2003) Six1 is required for the early organogenesis of mammalian kidney. Development 130:3085–3094
Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX (2003) The role of Six1 in mammalian auditory system development. Development 130:3989–4000
Chen R, Amoui M, Zhang Z, Mardon G (1997) Dachshund and Eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell 91:893–903
Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL (1997) The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell 91:881–891
Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ (1998) Eyeless initiates the expression of both sine oculis and Eyes absent during Drosophila compound eye development. Development 125:2181–2191
Halder G, Callaerts P, Gehring WJ (1995) Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 267:1788–1792
Borsani G, DeGrandi A, Ballabio A, Bulfone A, Bernard L, Banfi S, Gattuso C, Mariani M, Dixon M, Donnai D, Metcalfe K, Winter R, Robertson M, Axton R, Brown A, van Heyningen V, Hanson I (1999) EYA4, a novel vertebrate gene related to Drosophila Eyes absent. Hum Mol Genet 8:11–23
Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BN, Hartenstein V, Zipursky SL, Gruss P (1995) Homeobox genes and connective tissue patterning. Development 121:693–705
Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P (1995) Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 121:4045–4055
Xu PX, Cheng J, Epstein JA, Maas RL (1997) Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc Natl Acad Sci USA 94:11974–11979
Rayapureddi JP, Kattamuri C, Steinmetz BD, Frankfort BJ, Ostrin EJ, Mardon G, Hegde RS (2003) Eyes absent represents a class of protein tyrosine phosphatases. Nature 426:295–298
Tootle TL, Silver SJ, Davies EL, Newman V, Latek RR, Mills IA, Selengut JD, Parlikar BE, Rebay I (2003) The transcription factor Eyes absent is a protein tyrosine phosphatase. Nature 426:299–302
Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG (2003) Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature 426:247–254
Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG (2009) Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 458:591–596
Krishnan N, Jeong DG, Jung SK, Ryu SE, Xiao A, Allis CD, Kim SJ, Tonks NK (2009) Dephosphorylation of the C-terminal tyrosyl residue of the DNA damage-related histone H2A.X is mediated by the protein phosphatase Eyes absent. J Biol Chem 284:16066–16070
Kawakami K, Ohto H, Ikeda K, Roeder RG (1996) Structure, function and expression of a murine homeobox protein AREC3, a homologue of Drosophila sine oculis gene product, and implication in development. Nucleic Acids Res 24:303–310
Kawakami K, Ohto H, Takizawa T, Saito T (1996) Identification and expression of six family genes in mouse retina. FEBS Lett 393:259–263
Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, Maire P (1998) Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci USA 95:14220–14225
Suh CS, Ellingsen S, Austbo L, Zhao XF, Seo HC, Fjose A (2010) Autoregulatory binding sites in the zebrafish six3a promoter region define a new recognition sequence for Six3 proteins. FEBS J 277:1761–1775
Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K (1999) Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol 19:6815–6824
Silver SJ, Davies EL, Doyon L, Rebay I (2003) Functional dissection of Eyes absent reveals new modes of regulation within the retinal determination gene network. Mol Cell Biol 23:5989–5999
Li X, Perissi V, Liu F, Rose DW, Rosenfeld MG (2002) Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science 297:1180–1183
Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX (2010) Eya1-six1 interaction is sufficient to induce hair cell fate in the cochlea by activating atoh1 expression in cooperation with sox2. Dev Cell 22:377–390
Ahmed M, Xu J, Xu PX (2012) EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development 139:1965–1977
Ozaki H, Watanabe Y, Ikeda K, Kawakami K (2002) Impaired interactions between mouse Eyal harboring mutations found in patients with branchio-oto-renal syndrome and Six, Dach, and G proteins. J Hum Genet 47:107–116
Embry AC, Glick JL, Linder ME, Casey PJ (2004) Reciprocal signaling between the transcriptional co-factor Eya2 and specific members of the Galphai family. Mol Pharmacol 66:1325–1331
Fan X, Brass LF, Poncz M, Spitz F, Maire P, Manning DR (2000) The alpha subunits of Gz and Gi interact with the Eyes absent transcription cofactor Eya2, preventing its interaction with the six class of homeodomain-containing proteins. J Biol Chem 275:32129–32134
Landgraf K, Bollig F, Trowe MO, Besenbeck B, Ebert C, Kruspe D, Kispert A, Hanel F, Englert C (2010) Sipl1 and Rbck1 are novel Eya1-binding proteins with a role in craniofacial development. Mol Cell Biol 30:5764–5775
Nie X, Sun J, Gordon RE, Cai CL, Xu PX (2010) SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation. Development 137:755–765
Bonini NM, Leiserson WM, Benzer S (1998) Multiple roles of the Eyes absent gene in Drosophila. Dev Biol 196:42–57
Bane BC, Van Rybroek JM, Kolker SJ, Weeks DL, Manaligod JM (2005) EYA1 expression in the developing inner ear. Ann Otol Rhinol Laryngol 114:853–858
Buller C, Xu X, Marquis V, Schwanke R, Xu PX (2001) Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum Mol Genet 10:2775–2781
Zhang Y, Knosp BM, Maconochie M, Friedman RA, Smith RJ (2004) A comparative study of Eya1 and Eya4 protein function and its implication in branchio-oto-renal syndrome and DFNA10. J Assoc Res Otolaryngol 5:295–304
Xiong W, Dabbouseh NM, Rebay I (2009) Interactions with the Abelson tyrosine kinase reveal compartmentalization of Eyes absent function between nucleus and cytoplasm. Dev Cell 16:271–279
Okabe Y, Sano T, Nagata S (2009) Regulation of the innate immune response by threonine-phosphatase of Eyes absent. Nature 460:520–524
Pandey RN, Rani R, Yeo EJ, Spencer M, Hu S, Lang RA, Hegde RS (2010) The Eyes absent phosphatase-transactivator proteins promote proliferation, transformation, migration, and invasion of tumor cells. Oncogene 29:3715–3722
Chen B, Kim EH, Xu PX (2009) Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4. Dev Biol 326:75–85
Liu W, Lagutin OV, Mende M, Streit A, Oliver G (2006) Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J 25:5383–5395
Purcell P, Oliver G, Mardon G, Donner AL, Maas RL (2005) Pax6-dependence of Six3, Eya1 and Dach1 expression during lens and nasal placode induction. Gene Expr Patterns 6:110–118
Nica G, Herzog W, Sonntag C, Nowak M, Schwarz H, Zapata AG, Hammerschmidt M (2006) Eya1 is required for lineage-specific differentiation, but not for cell survival in the zebrafish adenohypophysis. Dev Biol 292:189–204
Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R (1999) Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet 23:113–117
Laclef C, Souil E, Demignon J, Maire P (2003) Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech Dev 120:669–679
Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, Okamura HO, Kitamura K, Muto S, Kotaki H, Sudo K, Horai R, Iwakura Y, Kawakami K (2004) Six1 controls patterning of the mouse otic vesicle. Development 131:551–562
Zou D, Silvius D, Fritzsch B, Xu PX (2004) Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development 131:5561–5572
Brugmann SA, Pandur PD, Kenyon KL, Pignoni F, Moody SA (2004) Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development 131:5871–5881
Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM Jr, Brophy PD, Berkman J, Gattas M, Hyland V, Ruf EM, Schwartz C, Chang EH, Smith RJ, Stratakis CA, Weil D, Petit C, Hildebrandt F (2004) SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci USA 101:8090–8095
Kozlowski DJ, Whitfield TT, Hukriede NA, Lam WK, Weinberg ES (2005) The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev Biol 277:27–41
Bricaud O, Collazo A (2006) The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J Neurosci 26:10438–10451
Schlosser G (2005) Evolutionary origins of vertebrate placodes: insights from developmental studies and from comparisons with other deuterostomes. J Exp Zoolog B Mol Dev Evol 304:347–399
Schlosser G (2006) Induction and specification of cranial placodes. Dev Biol 294:303–351
Li Y, Manaligod JM, Weeks DL (2010) EYA1 mutations associated with the branchio-oto-renal syndrome result in defective otic development in Xenopus laevis. Biol Cell 102:277–292
Hoskins BE, Cramer CH, Silvius D, Zou D, Raymond RM, Orten DJ, Kimberling WJ, Smith RJ, Weil D, Petit C, Otto EA, Xu PX, Hildebrandt F (2007) Transcription factor SIX5 is mutated in patients with branchio-oto-renal syndrome. Am J Hum Genet 80:800–804
Vincent C, Kalatzis V, Abdelhak S, Chaib H, Compain S, Helias J, Vaneecloo FM, Petit C (1997) BOR and BO syndromes are allelic defects of EYA1. Eur J Hum Genet 5:242–246
Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M (2000) Mutations of a human homologue of the Drosophila Eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet 9:363–366
Shimasaki N, Watanabe K, Hara M, Kosaki K (2004) EYA1 mutation in a newborn female presenting with cardiofacial syndrome. Pediatr Cardiol 25:411–413
Rayapureddi JP, Hegde RS (2006) Branchio-oto-renal syndrome associated mutations in Eyes absent 1 result in loss of phosphatase activity. FEBS Lett 580:3853–3859
Zou D, Silvius D, Rodrigo-Blomqvist S, Enerback S, Xu PX (2006) Eya1 regulates the growth of otic epithelium and interacts with Pax2 during the development of all sensory areas in the inner ear. Dev Biol 298:430–441
Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB (1998) Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc Natl Acad Sci USA 95:12608–12613
Coletta RD, Christensen K, Reichenberger KJ, Lamb J, Micomonaco D, Huang L, Wolf DM, Muller-Tidow C, Golub TR, Kawakami K, Ford HL (2004) The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci USA 101:6478–6483
Yu Y, Davicioni E, Triche TJ, Merlino G (2006) The homeoprotein six1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Cancer Res 66:1982–1989
Ozaki H, Watanabe Y, Takahashi K, Kitamura K, Tanaka A, Urase K, Momoi T, Sudo K, Sakagami J, Asano M, Iwakura Y, Kawakami K (2001) Six4, a putative myogenin gene regulator, is not essential for mouse embryonal development. Mol Cell Biol 21:3343–3350
Konishi Y, Ikeda K, Iwakura Y, Kawakami K (2006) Six1 and Six4 promote survival of sensory neurons during early trigeminal gangliogenesis. Brain Res 1116:93–102
Grifone R, Demignon J, Houbron C, Souil E, Niro C, Seller MJ, Hamard G, Maire P (2005) Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development 132:2235–2249
Wayne S, Robertson NG, DeClau F, Chen N, Verhoeven K, Prasad S, Tranebjarg L, Morton CC, Ryan AF, Van Camp G, Smith RJ (2001) Mutations in the transcriptional activator EYA4 cause late-onset deafness at the DFNA10 locus. Hum Mol Genet 10:195–200
Pfister M, Toth T, Thiele H, Haack B, Blin N, Zenner HP, Sziklai I, Nurnberg P, Kupka S (2002) A 4-bp insertion in the eya-homologous region (eyaHR) of EYA4 causes hearing impairment in a Hungarian family linked to DFNA10. Mol Med 8:607–611
Schonberger J, Wang L, Shin JT, Kim SD, Depreux FF, Zhu H, Zon L, Pizard A, Kim JB, Macrae CA, Mungall AJ, Seidman JG, Seidman CE (2005) Mutation in the transcriptional coactivator EYA4 causes dilated cardiomyopathy and sensorineural hearing loss. Nat Genet 37:418–422
Abe Y, Oka A, Mizuguchi M, Igarashi T, Ishikawa S, Aburatani H, Yokoyama S, Asahara H, Nagao K, Yamada M, Miyashita T (2009) EYA4, deleted in a case with middle interhemispheric variant of holoprosencephaly, interacts with SIX3 both physically and functionally. Hum Mutat 30:E946–E955
Cohen MM Jr (2006) Holoprosencephaly: clinical, anatomic, and molecular dimensions. Birth Defects Res A Clin Mol Teratol 76:658–673
Ribeiro LA, El-Jaick KB, Muenke M, Richieri-Costa A (2006) SIX3 mutations with holoprosencephaly. Am J Med Genet A 140:2577–2583
Christensen KL, Patrick AN, McCoy EL, Ford HL (2008) The six family of homeobox genes in development and cancer. Adv Cancer Res 101:93–126
Miller SJ, Lan ZD, Hardiman A, Wu J, Kordich JJ, Patmore DM, Hegde RS, Cripe TP, Cancelas JA, Collins MH, Ratner N (2010) Inhibition of Eyes Absent Homolog 4 expression induces malignant peripheral nerve sheath tumor necrosis. Oncogene 29:368–379
Wang CA, Jedlicka P, Patrick AN, Micalizzi DS, Lemmer KC, Deitsch E, Casas-Selves M, Harrell JC, Ford HL (2012) SIX1 induces lymphangiogenesis and metastasis via upregulation of VEGF-C in mouse models of breast cancer. J Clin Invest 122:1895–1906
Wang QF, Wu G, Mi S, He F, Wu J, Dong J, Luo RT, Mattison R, Kaberlein JJ, Prabhakar S, Ji H, Thirman MJ (2011) MLL fusion proteins preferentially regulate a subset of wild-type MLL target genes in the leukemic genome. Blood 117:6895–6905
Spitz F, Demignon J, Demeurie J, Sabourin JC, Kahn A, Daegelen D, Maire P (1998) A binding site for nuclear receptors is required for the differential expression of the aldolase A fast-twitch muscle promoter in body and head muscles. J Biol Chem 273:561–567
Grifone R, Laclef C, Spitz F, Lopez S, Demignon J, Guidotti JE, Kawakami K, Xu PX, Kelly R, Petrof BJ, Daegelen D, Concordet JP, Maire P (2004) Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype. Mol Cell Biol 24:6253–6267
Giordani J, Bajard L, Demignon J, Daubas P, Buckingham M, Maire P (2007) Six proteins regulate the activation of Myf5 expression in embryonic mouse limbs. Proc Natl Acad Sci USA 104:11310–11315
Dressler GR (2006) The cellular basis of kidney development. Annu Rev Cell Dev Biol 22:509–529
Kuure S, Vuolteenaho R, Vainio S (2000) Kidney morphogenesis: cellular and molecular regulation. Mech Dev 92:31–45
Saxen L, Sariola H, Lehtonen E (1986) Sequential cell and tissue interactions governing organogenesis of the kidney. Anat Embryol (Berl) 175:1–6
Saxen L, Sariola H (1987) Early organogenesis of the kidney. Pediatr Nephrol 1:385–392
Vainio S, Lin Y (2002) Coordinating early kidney development: lessons from gene targeting. Nat Rev Genet 3:533–543
Davies JA, Fisher CE (2002) Genes and proteins in renal development. Exp Nephrol 10:102–113
Costantini F (2006) Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation 74:402–421
Costantini F, Shakya R (2006) GDNF/Ret signaling and the development of the kidney. Bioessays 28:117–127
Dressler GR (2009) Advances in early kidney specification, development and patterning. Development 136:3863–3874
Sajithlal G, Zou D, Silvius D, Xu PX (2005) Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol 284:323–336
Mugford JW, Sipila P, McMahon JA, McMahon AP (2008) Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol 324:88–98
Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, Asai N, Takahashi M, Ohgami N, Kato M, Mendelsohn C, Costantini F (2009) Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell 17:199–209
Fraser FC, Sproule JR, Halal F (1980) Frequency of the branchio-oto-renal (BOR) syndrome in children with profound hearing loss. Am J Med Genet 7:341–349
Heimler A, Lieber E (1986) Branchio-oto-renal syndrome: reduced penetrance and variable expressivity in four generations of a large kindred. Am J Med Genet 25:15–27
Chen A, Francis M, Ni L, Cremers CW, Kimberling WJ, Sato Y, Phelps PD, Bellman SC, Wagner MJ, Pembrey M, Smith RJH (1995) Phenotypic manifestations of branchio-oto-renal syndrome. Am J Med Genet 58:365–370
Kobayashi H, Kawakami K, Asashima M, Nishinakamura R (2007) Six1 and Six4 are essential for Gdnf expression in the metanephric mesenchyme and ureteric bud formation, while Six1 deficiency alone causes mesonephric-tubule defects. Mech Dev 124:290–303
Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G (2006) Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25:5214–5228
Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP (2008) Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3:169–181
Weber S, Taylor JC, Winyard P, Baker KF, Sullivan-Brown J, Schild R, Knuppel T, Zurowska AM, Caldas-Alfonso A, Litwin M, Emre S, Ghiggeri GM, Bakkaloglu A, Mehls O, Antignac C, Network E, Schaefer F, Burdine RD (2008) SIX2 and BMP4 mutations associate with anomalous kidney development. J Am Soc Nephrol 19:891–903
Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D (2007) Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development 134:1967–1975
Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I (2000) Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105:863–873
Bush KT, Vaughn DA, Li X, Rosenfeld MG, Rose DW, Mendoza SA, Nigam SK (2006) Development and differentiation of the ureteric bud into the ureter in the absence of a kidney collecting system. Dev Biol 298:571–584
Nie X, Xu J, El-Hashash A, Xu PX (2011) Six1 regulates Grem1 expression in the metanephric mesenchyme to initiate branching morphogenesis. Dev Biol 351:141–151
Pauli T, Seimiya M, Blanco J, Gehring WJ (2005) Identification of functional sine oculis motifs in the autoregulatory element of its own gene, in the eyeless enhancer and in the signalling gene hedgehog. Development 132:2771–2782
Inaba M, Yuan H, Yamashita YM (2011) String (Cdc25) regulates stem cell maintenance, proliferation and aging in Drosophila testis. Development 138:5079–5086
Yan H, Canon J, Banerjee U (2003) A transcriptional chain linking eye specification to terminal determination of cone cells in the Drosophila eye. Dev Biol 263:323–329
Brodbeck S, Besenbeck B, Englert C (2004) The transcription factor Six2 activates expression of the Gdnf gene as well as its own promoter. Mech Dev 121:1211–1222
Himeda CL, Ranish JA, Angello JC, Maire P, Aebersold R, Hauschka SD (2004) Quantitative proteomic identification of six4 as the trex-binding factor in the muscle creatine kinase enhancer. Mol Cell Biol 24:2132–2143
Ando Z, Sato S, Ikeda K, Kawakami K (2005) Slc12a2 is a direct target of two closely related homeobox proteins, Six1 and Six4. FEBS J 272:3026–3041
Airik R, Bussen M, Singh MK, Petry M, Kispert A (2006) Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest 116:663–674
Acknowledgments
The author wishes to thank all of the past and present members of my lab for their many contributions and valuable discussions. Some of the work presented was supported by grants from the National Institutes of Health to P-X. X (NIH RO1 DC005824 and DK064640).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, PX. The EYA-SO/SIX complex in development and disease. Pediatr Nephrol 28, 843–854 (2013). https://doi.org/10.1007/s00467-012-2246-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-012-2246-1