Abstract
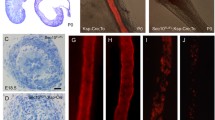
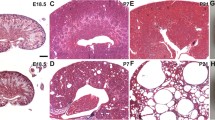
Congenital urinary tract obstruction is a major cause of progressive renal disease in children. We developed a model of partial unilateral ureteral obstruction (UUO) in the neonatal mouse, in which nephrogenesis at birth is similar to that of the midtrimester human fetus. The proximal tubule responds to UUO by undergoing apoptosis and necrosis, likely due to mitochondrial sensitivity to hypoxia and reactive oxygen species in the face of reduced endogenous antiapoptotic factors such as eNOS. Damage to the glomerulotubular junction is followed by scission and formation of atubular glomeruli and aglomerular tubules. This is an orchestrated process, with atubular glomeruli surrounded by a continuous layer of regenerated parietal epithelial cells. Relief of UUO at 7 days of age results in remodeling of the renal parenchyma by adulthood. In contrast to proximal tubular destruction, collecting ducts remain dilated and patent, with remodeling due to apoptosis and proliferation (a process associated with recruitment of intercalated cells as progenitor cells following UUO in the fetal monkey). Formation of atubular glomeruli occurs in other renal disorders (congenital nephrotic syndrome and cystinosis), and may represent a maladaptive response to proximal tubular injury reflecting an evolutionary adaptation by an ancestor we share with aglomerular marine fish.



Similar content being viewed by others
References
Thornhill BA, Forbes MS, Marcinko ES, Chevalier RL (2007) Glomerulotubular disconnection in neonatal mice after relief of partial ureteral obstruction. Kidney Int 72:1103–1112
Chevalier RL, Thornhill BA, Forbes MS, Kiley SC (2010) Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol 25:687–697
Hall AM, Unwin RJ, Parker N, Duchen MR (2009) Multiphoton imaging reveals differences in mitochondrial function between nephron segments. J Am Soc Nephrol 20:1293–1302
Ohse T, Pippin JW, Chang AM, Krofft RD, Miner JH, Vaughan MR, Shankland SJ (2009) The enigmatic parietal epithelial cell is finally getting noticed: a review. Kidney Int 76:1225–1238
Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P (2009) Regeneration of glomerular podocytes by human renal progenitors [see comment]. J Am Soc Nephrol 20:322–332
Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumanr P, Bidani AK (2010) Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol 298:F1078–F1094
Chevalier RL, Forbes MS (2008) Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol 19:197–206
Forbes MS, Thornhill BA, Park MH, Chevalier RL (2007) Lack of endothelial nitric-oxide synthase leads to progressive focal renal injury. Am J Pathol 170:87–99
Silverstein DM, Travis BR, Thornhill BA, Schurr JS, Kolls JK, Leung JC, Chevalier RL (2003) Altered expression of immune modulator and structural genes in neonatal unilateral ureteral obstruction. Kidney Int 64:25–35
Valles P, Pascual L, Manucha W, Carrizo L, Ruttler M (2003) Role of endogenous nitric oxide in unilateral ureteropelvic junction obstruction in children. Kidney Int 63:1104–1115
Butt MJ, Tarantal AF, Jimenez DF, Matsell DG (2007) Collecting duct epithelial-mesenchymal transition in fetal urinary tract obstruction. Kidney Int 72:936–944
Konda R, Orikasa S, Sakai K, Ota S, Kimura N (1996) The distribution of renin containing cells in scarred kidneys. J Urol 156:1450–1454
El-Dahr SS, Gomez RA, Gray MS, Peach MJ, Carey RM, Chevalier RL (1990) In situ localization of renin and its mRNA in neonatal ureteral obstruction. Am J Physiol 258:F854–F862
Cohen EP, Regner K, Fish BL, Moulder JE (2000) Stenotic glomerulotubular necks in radiation nephropathy. J Pathol 190:484–488
Benigni A, Gagliardini E, Remuzzi A, Corna D, Remuzzi G (2001) Angiotensin-converting enzyme inhibition prevents glomerular-tubule disconnection and atrophy in passive Heymann nephritis, an effect not observed with a calcium antagonist. Am J Pathol 159:1743–1750
Marcussen N, Olsen TS (1990) Atubular glomeruli in patients with chronic pyelonephritis. Lab Invest 62:467–473
Thoene JG (2007) A review of the role of enhanced apoptosis in the pathophysiology of cystinosis. Molec Genet Metab 92:292–298
Vats AN, Costello B, Mauer M (2003) Glomerular structural factors in progression of congenital nephrotic syndrome. Pediatr Nephrol 18:234–240
Najafian B, Kim Y, Crosson JT, Mauer M (2003) Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. J Am Soc Nephrol 14:908–917
Brezniceanu M-L, Liu F, Wei C-C, Tran S, Sachetelli S, Zhang S-L, Guo D-F, Filep JG, Ingelfinger JR, Chan JSD (2007) Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int 71:912–923
Oliver J, Luey AB (1935) Plastic studies in abnormal renal architecture. Arch Pathol 19:1–23
Marcussen N (2000) Tubulointerstitial damage leads to atubular glomeruli: significance and possible role in progression. Nephrol Dial Transplant 15(Suppl 6):74–75
Thornhill BA, Burt LA, Chen C, Forbes MS, Chevalier RL (2005) Variable chronic partial ureteral obstruction in the neonatal rat: A new model of ureteropelvic junction obstruction. Kidney Int 67:42–52
Chevalier RL, Thornhill BA, Chang AY (2000) Unilateral ureteral obstruction in neonatal rats leads to renal insufficiency in adulthood. Kidney Int 58:1987–1995
Grantham JJ, Wallace DP (2002) Return of the secretory kidney. Am J Physiol 282:F1–F9
Smith HW (1953) From fish to philosopher. Little, Brown, Boston
Grafflin AL (1933) Glomerular degeneration in the kidney of the daddy sculpin (Myoxocephalus scorpius). Anat Rec 57:59–78
Beyenbach KW (2003) Kidneys sans glomeruli. Am J Physiol 286:F811–F827
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chevalier, R.L., Forbes, M.S. & Thornhill, B.A. Formation of atubular glomeruli in the developing kidney following chronic urinary tract obstruction. Pediatr Nephrol 26, 1381–1385 (2011). https://doi.org/10.1007/s00467-010-1748-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1748-y