Abstract
Nephrolithiasis is a cause of significant morbidity and medical care expenses worldwide. Its prevalence has increased steadily during the last three decades among both adults and children. This trend suggests that changing environmental factors play a significant role in the risk of developing kidney stones although, conversely, there are many indications that genes play an important role in this condition as well. A limited number of monogenic forms have been identified, but the majority of nephrolithiasis cases are the result of complex, multi-factorial interactions between genetic inheritance and environmental exposure. Scientific evidence indicates that inheritance accounts for about half of the risk of common forms, making these forms suitable for investigation by genetic analysis. Here, we review the numerous studies that have been conducted to establish the role of genes in determining the risk of nephrolithiasis, the differential contribution of genes to this risk, and the confounding influence that environmental variables have on genetic studies.
Similar content being viewed by others
References
Pak CY (1998) Kidney stones. Lancet 351:1797–1801
Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC (2003) Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 63:1817–1823
Trinchieri A, Ostini F, Nespoli R, Rovera F, Montanari E, Zanetti G (1999) A prospective study of recurrence rate and risk factors for recurrence after a first renal stone. J Urol 162:27–30
Pearle MS, Calhoun E, Curhan GC (2007) Urolithiasis. In: Litwin MS, Saigal CS (eds) Urologic diseases in America. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. NIH Publication No. 07-5512. US Government Printing Office, Washington D.C., pp 282–319
Moe OW, Bonny O (2005) Genetic hypercalciuria. J Am Soc Nephrol 16:729–745
Gambaro G, Vezzoli G, Casari G, Rampoldi L, D’Angelo A, Borghi L (2004) Genetics of hypercalciuria and calcium nephrolithiasis: from the rare monogenic to the common polygenic forms. Am J Kidney Dis 44:963–986
Stechman MJ, Loh NY, Thakker RV (2007) Genetics of hypercalciuric nephrolithiasis: renal stone disease. Ann N Y Acad Sci 1116:461–484
Robertson WG (2003) Renal stones in the tropics. Semin Nephrol 23:77–87
Soucie JM, Coates RJ, McClellan W, Austin H, Thun M (1996) Relation between geographic variability in kidney stones prevalence and risk factors for stones. Am J Epidemiol 143:487–495
Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H (1994) Demographic and geographic variability of kidney stones in the United States. Kidney Int 46:893–899
Curhan GC (1997) Dietary calcium, dietary protein, and kidney stone formation. Miner Electrolyte Metab 23:261–264
Mente A, Honey RJ, McLaughlin JR, Bull SB, Logan AG (2007) Ethnic differences in relative risk of idiopathic calcium nephrolithiasis in North America. J Urol 178:1992–1997, discussion 1997
Cosman F, Nieves J, Dempster D, Lindsay R (2007) Vitamin D economy in blacks. J Bone Miner Res 22[Suppl 2]:V34–38
Taylor EN, Curhan GC (2007) Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol 18:654–659
Braun M, Palacios C, Wigertz K, Jackman LA, Bryant RJ, McCabe LD, Martin BR, McCabe GP, Peacock M, Weaver CM (2007) Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr 85:1657–1663
Curhan GC, Willett WC, Rimm EB, Stampfer MJ (1997) Family history and risk of kidney stones. J Am Soc Nephrol 8:1568–1573
Coe FL, Parks JH, Moore ES (1979) Familial idiopathic hypercalciuria. N Engl J Med 300:337–340
Nicolaidou P, Themeli S, Karpathios T, Georgouli H, Athanassaki K, Xaidara A, Messaritakis J (1996) Family pattern of idiopathic hypercalciuria and its subtypes. J Urol 155:1042–1044
Visscher PM, Medland SE, Ferreira MA, Morley KI, Zhu G, Cornes BK, Montgomery GW, Martin NG (2006) Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genet 2:e41
McGeown MG (1960) Heredity in renal stone disease. Clin Sci 19:465–471
Griffin DG (2004) A review of the heritability of idiopathic nephrolithiasis. J Clin Pathol 57:793–796
Trinchieri A, Mandressi A, Luongo P, Coppi F, Pisani E (1988) Familial aggregation of renal calcium stone disease. J Urol 139:478–481
Resnick M, Pridgen DB, Goodman HO (1968) Genetic predisposition to formation of calcium oxalate renal calculi. N Engl J Med 278:1313–1318
Falconer GF, Adams CW (1965) The relationship between nutritional state and severity of atherosclerosis. Guys Hosp Rep 114:130–139
Goldfarb DS, Fischer ME, Keich Y, Goldberg J (2005) A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int 67:1053–1061
Wolf MT, Zalewski I, Martin FC, Ruf R, Muller D, Hennies HC, Schwarz S, Panther F, Attanasio M, Acosta HG, Imm A, Lucke B, Utsch B, Otto E, Nurnberg P, Nieto VG, Hildebrandt F (2005) Mapping a new suggestive gene locus for autosomal dominant nephrolithiasis to chromosome 9q33.2-q34.2 by total genome search for linkage. Nephrol Dial Transplant 20:909–914
Reed BY, Heller HJ, Gitomer WL, Pak CY (1999) Mapping a gene defect in absorptive hypercalciuria to chromosome 1q23.3-q24. J Clin Endocrinol Metab 84:3907–3913
Edwards JH (1960) The simulation of Mendelism. Acta Genet Stat Med 10:63–70
Goodman HO, Holmes RP, Assimos DG (1996) Re: family pattern of idiopathic hypercalciuria and its subtypes. J Urol 156:2012
Loredo-Osti JC, Roslin NM, Tessier J, Fujiwara TM, Morgan K, Bonnardeaux A (2005) Segregation of urine calcium excretion in families ascertained for nephrolithiasis: evidence for a major gene. Kidney Int 68:966–971
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316:889–894
Devuyst O, Pirson Y (2007) Genetics of hypercalciuric stone forming diseases. Kidney Int 72:1065–1072
Muldowney FP, Freaney R, Moloney MF (1982) Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int 22:292–296
Stapleton FB (2002) Childhood stones. Endocrinol Metab Clin North Am 31:1001–1015
Cirillo M, Stellato D, Panarelli P, Laurenzi M, De Santo NG (2003) Cross-sectional and prospective data on urinary calcium and urinary stone disease. Kidney Int 63:2200–2206
Hodgkinson A, Pyrah LN (1958) The urinary excretion of calcium and inorganic phosphate in 344 patients with calcium stone of renal origin. Br J Surg 46:10–18
Curhan GC, Willett WC, Speizer FE, Stampfer MJ (2001) Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int 59:2290–2298
Hunter DJ, Lange M, Snieder H, MacGregor AJ, Swaminathan R, Thakker RV, Spector TD (2002) Genetic contribution to renal function and electrolyte balance: a twin study. Clin Sci (Lond) 103:259–265
Hunter D, De Lange M, Snieder H, MacGregor AJ, Swaminathan R, Thakker RV, Spector TD (2001) Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res 16:371–378
Osorio AV, Alon US (1997) The relationship between urinary calcium, sodium, and potassium excretion and the role of potassium in treating idiopathic hypercalciuria. Pediatrics 100:675–681
So NP, Osorio AV, Simon SD, Alon US (2001) Normal urinary calcium/creatinine ratios in African-American and Caucasian children. Pediatr Nephrol 16:133–139
Johnson CM, Wilson DM, O’Fallon WM, Malek RS, Kurland LT (1979) Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int 16:624–631
Holmes RP, Assimos DG, Goodman HO (1998) Genetic and dietary influences on urinary oxalate excretion. Urol Res 26:195–200
Hamm LL, Hering-Smith KS (2002) Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol Metab Clin North Am 31:885–893, viii
Shah O, Assimos DG, Holmes RP (2005) Genetic and dietary factors in urinary citrate excretion. J Endourol 19:177–182
Bushinsky DA (1996) Genetic hypercalciuric stone forming rats. Semin Nephrol 16:448–457
Hoopes RR Jr, Reid R, Sen S, Szpirer C, Dixon P, Pannett AA, Thakker RV, Bushinsky DA, Scheinman SJ (2003) Quantitative trait loci for hypercalciuria in a rat model of kidney stone disease. J Am Soc Nephrol 14:1844–1850
Asplin JR, Bauer KA, Kinder J, Muller G, Coe BJ, Parks JH, Coe FL (2003) Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int 63:662–669
Garcia-Nieto V, Ferrandez C, Monge M, de Sequera M, Rodrigo MD (1997) Bone mineral density in pediatric patients with idiopathic hypercalciuria. Pediatr Nephrol 11:578–583
Reed BY, Gitomer WL, Heller HJ, Hsu MC, Lemke M, Padalino P, Pak CY (2002) Identification and characterization of a gene with base substitutions associated with the absorptive hypercalciuria phenotype and low spinal bone density. J Clin Endocrinol Metab 87:1476–1485
Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d’Ancona FC, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K (2009) Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41:926–930
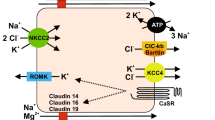
Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP (1999) Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285:103–106
Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S (2006) Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79:949–957
Taylor EN, Stampfer MJ, Curhan GC (2005) Obesity, weight gain, and the risk of kidney stones. JAMA 293:455–462
Mente A, Honey RJ, McLaughlin JM, Bull SB, Logan AG (2006) High urinary calcium excretion and genetic susceptibility to hypertension and kidney stone disease. J Am Soc Nephrol 17:2567–2575
Pak CY, Sakhaee K, Moe O, Preminger GM, Poindexter JR, Peterson RD, Pietrow P, Ekeruo W (2003) Biochemical profile of stone-forming patients with diabetes mellitus. Urology 61:523–527
Lloyd SE, Pearce SH, Fisher SE, Steinmeyer K, Schwappach B, Scheinman SJ, Harding B, Bolino A, Devoto M, Goodyer P, Rigden SP, Wrong O, Jentsch TJ, Craig IW, Thakker RV (1996) A common molecular basis for three inherited kidney stone diseases. Nature 379:445–449
Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP (1996) Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13:183–188
Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP (1996) Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14:152–156
Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP (1997) Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet 17:171–178
Hendy GN, Minutti C, Canaff L, Pidasheva S, Yang B, Nouhi Z, Zimmerman D, Wei C, Cole DE (2003) Recurrent familial hypocalcemia due to germline mosaicism for an activating mutation of the calcium-sensing receptor gene. J Clin Endocrinol Metab 88:3674–3681
Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaître X, Paillard M, Planelles G, Déchaux M, Miller RT, Antignac C (2002) Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol 13:2259–2266
Bruce LJ, Cope DL, Jones GK, Schofield AE, Burley M, Povey S, Unwin RJ, Wrong O, Tanner MJ (1997) Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Invest 100:1693–1707
Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE (2000) Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26:71–75
Nishiyama K, Funai T, Katafuchi R, Hattori F, Onoyama K, Ichiyama A (1991) Primary hyperoxaluria type I due to a point mutation of T to C in the coding region of the serine:pyruvate aminotransferase gene. Biochem Biophys Res Commun 176:1093–1099
Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP (1999) The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II. Hum Mol Genet 8:2063–2069
Parvari R, Brodyansky I, Elpeleg O, Moses S, Landau D, Hershkovitz E (2001) A recessive contiguous gene deletion of chromosome 2p16 associated with cystinuria and a mitochondrial disease. Am J Hum Genet 69:869–875
Feliubadaló L, Font M, Purroy J, Rousaud F, Estivill X, Nunes V, Golomb E, Centola M, Aksentijevich I, Kreiss Y, Goldman B, Pras M, Kastner DL, Pras E, Gasparini P, Bisceglia L, Beccia E, Gallucci M, de Sanctis L, Ponzone A, Rizzoni GF, Zelante L, Bassi MT, George AL Jr, Manzoni M, De Grandi A, Riboni M, Endsley JK, Ballabio A, Borsani G, Reig N, Fernández E, Estévez R, Pineda M, Torrents D, Camps M, Lloberas J, Zorzano A, Palacín M; International Cystinuria Consortium (1999) Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo,+AT) of rBAT. Nat Genet 23:52–57
Monga M, Macias B, Groppo E, Hargens A (2006) Genetic heritability of urinary stone risk in identical twins. J Urol 175:2125–2128
Piwon N, Günther W, Schwake M, Bösl MR, Jentsch TJ (2000) ClC-5 Cl- -channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature 408:369–373
Cao G, Yang G, Timme TL, Saika T, Truong LD, Satoh T, Goltsov A, Park SH, Men T, Kusaka N, Tian W, Ren C, Wang H, Kadmon D, Cai WW, Chinault AC, Boone TB, Bradley A, Thompson TC (2003) Disruption of the caveolin-1 gene impairs renal calcium reabsorption and leads to hypercalciuria and urolithiasis. Am J Pathol 162:1241–1248
Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS (2006) Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38:474–478
Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS (1998) Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A 95:5372–5377
Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Mérillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ (2003) Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112:1906–1914
Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, Cornell RA, Parichy DM (2005) Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr Biol 15:667–671
Bannasch D, Safra N, Young A, Karmi N, Schaible RS, Ling GV (2008) Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet 4:e1000246
Author information
Authors and Affiliations
Corresponding author
Glossary
- Linkage analysis
-
A technique that allows one to assign to genomic regions identified by unique markers the probability to be causative of an observed phenotype. It is performed by comparing the observed cosegregation of markers and phenotypes within one or more pedigrees to the null hypothesis of no cosegregation.
- Effect size
-
The extent of the effect of a locus in determining a trait. An effect size of 1 would correspond to a full penetrant allele completely determining the trait.
- Endo-phenotype
-
An inheritable trait associated with a disease in the population and co-segregating with the disease in pedigrees that is detectable whether or not the disease phenotype is manifest.
- Relative risk (λ)
-
The ratio of the prevalence of a disease in a risk population to the prevalence of the same disease in the general population (λ = R R /R P ). It is a measure of familiarity for a certain condition when the risk population is represented by the relatives of diseased individuals. Notice that this index is not only influenced by genetic factors but also by any other factor shared in the family.
- Heritability (h 2)
-
The ratio of the variability determined by additive genetic effects on the overall phenotypic variability (h 2 = σ A /σ P ). Heritability is a stricter index of genetic contribution to a trait. It does not exclude the existence of non-inheritable components but can never be higher than the overall genetic risk, representing a better estimate of the genetic substratum. A heritability of 0.5 indicates that at least 50% of the familiarity of a trait is explained by inheritable genetic factors.
- Regression
-
A statistics that quantifies how the variance of a dependent variable is explained by changes in the independent variable.
- Twins concordance
-
The conditional probability of the second twin expressing a trait given that the first twin expresses that trait. The concordance in a phenotype between monozygotic twins (MZ, sharing 100% of their alleles) is expected to be higher than that between dizygotic twins (DZ, sharing 50% of their alleles). The ratio of MZ/DZ concordance for a trait can be used as measure of genetic influence on that trait. Although it is still subject to shared pre- and postnatal environment, it is a more reliable index of genetic contribution. High MZ/DZ concordance is strongly suggestive of the existence of heritable genetic risk.
- Penetrance
-
The probability, from zero to 1, of expressing the phenotype in the presence of the risk allele(s). The probability is 1 for Mendelian alleles with strong effect on the phenotype (fully penetrant). A lower than 1 probability can be explained by the concomitance of other variables.
- Quantitative Trait
-
Any trait measurable as a continuous variable (like height, blood pressure, etc.)
- Additive effect
-
In genetics, it refers to the effect that genes have on a trait based only on the number of inherited alleles, not on genetic interactions between genes or alleles, such as dominance (if one of the allele is present it will per se determine the trait) or gene–gene interactions.
- Quantitative linkage analysis
-
A variant of linkage analysis in which, instead of a dichotomous (affected/non affected) phenotype, the deviation of the value of a quantitative trait in a subject from the population mean is tested for cosegregation with genetic markers.
Rights and permissions
About this article
Cite this article
Attanasio, M. The genetic components of idiopathic nephrolithiasis. Pediatr Nephrol 26, 337–346 (2011). https://doi.org/10.1007/s00467-010-1562-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1562-6