Abstract
Intrauterine growth retardation is presumed to be associated with decreased renal size and impaired renal function as a result of stunted kidney development and nephron deficit. To study whether very preterm birth also affects renal size at young adulthood, we sonographically measured bipolar kidney length and volume in 51 very premature individuals (<32 weeks of gestation), either small (SGA) or appropriate (AGA) for gestational age (22 SGA and 29 AGA), and 30 full-term controls 20 years after birth. Relative kidney length and volume were calculated. Both absolute and relative left kidney length and volume were significantly lower in SGA and AGA individuals, notably in women. Renal size did not differ between SGA and AGA individuals. In 70% of controls, the left kidney was larger than the right one compared with 40.9% in SGA [relative risk (RR) 1.7; 95% confidence interval (CI) 1.0−3.0] and 48.3% in AGA (RR 1.5; 95% CI 0.9−2.3) individuals. Renal structural anomalies were present in eight prematurely born participants only. Our data suggest that kidney growth is stunted after preterm birth, especially on the left side, and in the female gender.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increasing number of studies show an association between intrauterine growth retardation (IUGR) and underdevelopment of the fetal kidneys, with limited nephron number and decreased renal size [1−4]. Furthermore, birth weight appears to be a strong determinant of renal size, nephron number, glomerular volume, albuminuria and systolic blood pressure [5, 6]. These findings add weight to the hypothesis that IUGR carries a risk of renal function loss as a result of nephron deficit, loss of filtration surface area, hyperfiltration, glomerular hypertension, and glomerular damage [7]. As nephrogenesis continues until 36 weeks of gestation, very preterm babies (gestational age <32 weeks) are likely to show a nephron deficit at birth [8]. In addition, preterm birth is known to be associated with impaired nephrogenesis [9, 10] and limited postnatal kidney growth until the age of 18–24 months [11, 12]. Whether adult renal size is also impaired after premature birth compared with full-term birth or whether renal catch-up growth during childhood is present has not yet been studied.
We report reduced renal size (length and volume) in 20-year-old individuals born very prematurely [13]. Renal size was correlated with glomerular filtration rate (GFR) (ml/min/1.73 m2) and effective renal plasma flow (ml/min/1.73 m2). The data reported here documents an additional analysis on renal size in the same cohort.
Methods
Study population
We recruited three groups of 20-year-olds: (1) Those born very prematurely (<32 weeks) and small for gestational age (SGA), (2) those born very prematurely and appropriate for gestational age (AGA), and (3) those born full term (37–42 weeks) and AGA (controls). The SGA and AGA groups participated in a follow-up study of almost all (94%) of live-born individuals in the Netherlands with a gestational age <32 weeks and/or a birth weight <1,500 g. This is the POPS cohort: Project on Prematures and Small for Gestational Age infants. Participants in the our study had participated in the most recent POPS 19 study (N = 422) [14]. Recruitment of SGA individuals started with the lowest birth weight adjusted for gestational age [≤ −2 standard deviation scores (SDS) N = 29] and that of AGA individuals with the highest birth weight adjusted for gestational age (0–2 SDS N = 205). Controls born between 1 January 1982 and 31 December 1984 were recruited by advertisement posted in the Erasmus Medical Center, Rotterdam, The Netherlands.
Renal ultrasound
Participants underwent renal function testing, blood pressure measurement, and renal ultrasound study in the Erasmus MC Sophia Children’s Hospital, Rotterdam [13]. Each of the three pediatric radiologists performed renal ultrasound by standardized protocol. Renal size was measured in the prone position with a Philips ATL HDI 5000 scanner with a C5–2 curved-array transducer (Philips Nederland BV, Eindhoven, The Netherlands). Measures of maximal bipolar kidney length, width, and thickness were obtained for both kidneys. Renal width and thickness were measured at the level of the kidney hilum. Renal volume (ml) was calculated using the formula for an ellipsoid: (π / 6) * length (cm) *width (cm) * thickness (cm) [15]. Cortical thickness was defined as the distance between the external surface of the kidney and top of the medulla. As absolute renal size is known to be associated with body size, we calculated the relative kidney length length (bipolar kidney length (cm) / body height (m)) and relative kidney volume (bipolar kidney length (ml) / body surface area (BSA, m2)) [16]. Body surface area (BSA) was calculated using the equation √ ((body height (m) * weight (kg)) / 3600). Any renal or ureterovesical anomalies noted were reported.
Intra- and interobserver variability
Intra- and interobserver variability for the three pediatric radiologists were calculated beforehand for measurements in seven healthy students (five females, two males). The mean (95% limits of agreement) intraobserver variability for bipolar renal length ranged between 0.0 cm (−1.1 to 1.1) and 0.1 cm (−1.0 to 1.2) for the left kidney and 0.1 cm (−0.7 to 0.9) and 0.4 cm (−0.5 to 1.4) for the right kidney. The intraclass coefficient (ICC) for intraobserver variability was 0.97 for left bipolar kidney length and 0.88 for right bipolar kidney length. The mean (95% limits of agreement) interobserver variability for bipolar renal length ranged between 0.0 cm (−1.4 to 1.5) and 0.2 cm (−1.0 to 1.5) for the left kidney and 0.1 cm (−0.1 to 0.3) and 0.2 cm (−0.4 to 0.8) for the right kidney. The ICC for interobserver variability was 0.84 for left bipolar kidney length and 0.86 for right bipolar kidney length. Renal volume estimates varied more widely, but significant systematic errors within and between observers did not occur.
Informed consent and ethics committee
Informed consent was obtained from all participants after oral and written information had been given. The Erasmus MC University Medical Center ethical review board approved the study protocol.
Statistics
Statistical analysis was performed with SPSS 15.0 software. Results are presented as mean and SD. One-way analysis of variance (ANOVA) with post hoc multiple comparisons (Bonferroni) was used to analyze group differences in baseline characteristics. Pearson’s chi-square tests were performed to compare proportions of males between study groups; Fisher exact test was used to compare prevalences of renal anomalies. Multivariate regression analysis was used to determine regression coefficients in the association between body height or BSA and renal length and volume. Statistical significance was considered at the level of 5%.
Results
Baseline characteristics of study population
Eighty-two individuals agreed to participate: i.e. 23 SGA individuals, 29 AGA individuals, and 30 controls. However, one SGA individual suffered an unrelated allergic episode prior to study and did not undergo renal ultrasound, reducing the SGA group to 22. Baseline characteristics are shown in Table 1. Birth weight and gestational age significantly differed between the three groups. Current weight, height, and BSA of SGA individuals were lower than those of AGA individuals and controls, but body mass index (BMI) did not differ between groups.
Renal size
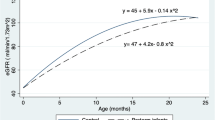
Renal size is detailed in Table 2. Male individuals had significantly larger kidney volume than female individuals (ANOVA p value < 0.05). Left kidneys were larger than right kidneys in 70% of controls, in 48.3% of AGA individuals [relative risk (RR) 1.5; 95% confidence interval (CI) 0.9−2.3], and in 40.9% of SGA individuals (RR 1.7; 95% CI 1.0−3.0) (Fig. 1). Total kidney volume in both SGA and AGA individuals was significantly smaller than that in controls (p < 0.05) (Table 2, Fig. 2). Also, absolute and relative left renal length and volume in SGA and AGA individuals were smaller than in controls. Right renal size did not differ between groups. Both left and right kidney size did not differ between SGA and AGA individuals. Cortical thickness did not differ between groups. Interestingly, after stratification for sex, all the above differences proved statistically significant in the female gender only.
Male and female individuals in the control group did not differ with regard to renal size for both kidneys. Male and female individuals in both the SGA and AGA groups differed statistically significantly with regard to total kidney volume (ANOVA p value < 0.05). BSA was positively related to volume [β 215 ml/m2 (95% CI 147−283); p < 0.001] as shown in Fig. 3a. Body height was positively related to renal length [β 0.09 cm/cm (95% CI 0.06−0.12); p < 0.001] as shown in Fig. 3b. In Fig. 4a–d, BSA and body height are plotted separately for the left and right kidney against kidney volume and length, respectively.
Renal and ureterovesical anomalies
Renal ultrasound revealed subclinical anomalies of the kidneys and ureters in eight of 51 individuals born prematurely (Table 3) and not in controls (Fisher exact test p value = 0.02). Anomalies varied from nephrocalcinosis (N = 1), unilateral pyelocaliceal dilatation (N = 3), ureteropelvic junction obstruction (N = 1), ureter dilatation (N = 1), extrarenal pelvis (N = 1), and ureter duplication and ureterocele (N = 1). These were not known before the ultrasound study. Prevalences of the anomalies did not differ between SGA (3/22) and AGA (5/29) individuals. Analyses excluding these eight individuals did not affect findings.
Discussion
Renal ultrasound study revealed significantly smaller renal length and volume in 20-year-old female individuals born very prematurely, either SGA or AGA, compared with age-matched controls. The difference was most pronounced for the left kidney. Differences were observed for male individuals also, but statistical significance was reached only in female individuals. IUGR had no significant effect on renal size. These results are consistent with impaired fetal kidney development after preterm birth, with probably no more than a small effect of IUGR on renal size. Nephrogenesis starts in early pregnancy, but it peaks at the 32nd week of gestation, continues until 36 weeks of gestation, and is correlated to renal size [5]. The AGA and SGA groups, both born before a gestational age of 32 weeks, did not differ in renal size, possibly because nephrogenesis was not complete at birth. We found differences in renal size between male and female individuals in both the SGA and AGA groups. Such gender differences have only been reported in an experimental IUGR rat model, suggestive of the importance of gender in outcomes in adulthood after IUGR [17]. In our study, differences in renal size between male SGA, AGA, and control individuals seemed smaller than those between female study participants but still showed a trend for decreased absolute renal length and volume. The lack of significance might be due to insufficient power of the study.
Singh et al. described that lower renal volume in Australian Aborigines represents kidneys with reduced nephron number [5]. As there is a limited postnatal nephrogenesis in preterm individuals [9, 10], a nephron deficit after preterm birth probably persists throughout life. From a study in 56 deceased extremely premature infants and ten deceased full-term controls, it appeared that nephron number was highly correlated to gestational age and that nephrogenesis had stopped 40 days postnatally [9]. As nephron deficit predisposes to reduced renal function in the adult [5, 6], preterm individuals are at such risk. Earlier, we found higher blood pressure and poor renal function and microalbuminuria in these premature individuals [14, 18]. Furthermore, restricted postnatal growth after premature birth may impair renal function over time [19].
Decreased renal volume was also found in 33 low-birth-weight (LBW) Aboriginal children between 5–18 years of age [3]. Their BSA-adjusted renal volume was 78.5 ml/m2 compared with 85.7 ml/m2 in 141 normal birth weight (NBW) individuals (p = 0.018). Renal length was equal. The authors stated that LBW resulted from IUGR, but data on gestational age were unavailable in many participants. For that matter, our study found that IUGR did not affect renal size. Renal size differences between preterms and controls were most pronounced for the left kidney, in line with findings in Aboriginal children [3]. Interestingly, hypertensive adults with chronic kidney failure showed smaller absolute left kidney volume (but not right kidney volume) compared with normotensive chronic kidney failure patients [20]. The authors did not study whether renal size in these hypertensive patients was associated with birth weight and gestational age.
Thus, from results of other studies and this study, it would seem that very preterm individuals show limited early postnatal renal growth, especially of the left kidney. Other studies reported that postnatal renal growth after preterm birth was limited until 18−24 months of age but did not mention differences in left and right kidney growth [11, 12]. To our knowledge, only one study described normal fetal kidney growth separately for the left and right kidney [21]. Sonography at several gestational ages did not reveal significant difference between left and right kidney growth between 16 and 38 weeks of gestation. We can only speculate that the left kidney grows more rapidly than the right kidney after the gestational age of 32 weeks in normal gestation, but large studies are needed to confirm this speculation.
Ultrasonography revealed renal anomalies in eight very preterm participants versus none of the controls. The anomalies mainly comprised ectasia or dilatation of the ureters and pyelocaliceal system. All individuals were asymptomatic. It is not known whether the anomalies were already present at birth. They may have developed in the early postnatal period or in childhood but then remained unnoticed, as no ultrasound studies were performed. Some renal diseases, such as acute tubular necrosis and nephrocalcinosis, occur more frequently in prematurely born children, but higher incidences of renal anomalies we found have not yet been reported [22, 23]. One possible biological pathway may be inferred from the observation that in-utero disturbance of the renin-angiotensin system affects normal kidney development [24, 25].
The strength of our study lies in evaluating the effect of prematurity separately from that of IUGR. The difference in renal size was most pronounced between the AGA preterm and full-term participants. The additional effect of IUGR in these very preterm subjects was small and not significant for all renal sizes. Therefore, we suggest that limited renal growth after very preterm birth predisposes to decreased renal size at adult age.
Two possible limitations of the study should be mentioned. First, selection bias may have been introduced by selecting students only as controls. Second, three different radiologists obtained the data of renal size only once. However, to minimize the variability between the three radiologists, renal measurements were made by a standardized protocol, and all radiologists measured subjects from all three groups. Inter- and intraobserver variability testing showed a slight mean error in measurement, which was comparable with other studies [26, 27]. Moreover, renal lengths measured in controls were similar to those reported by Miletic et al. [28], indicating the radiologists in our study produced accurate measurements.
In conclusion, kidney development after preterm birth is probably stunted, leading to (relatively) smaller kidneys and a higher frequency of renal structural anomalies at the age of 20 years. There is only a small, nonsignificant, additional effect of IUGR on these observations. The effects of stunted development were more obvious in left kidneys and in female individuals. Detailed measurement of renal development, in both size and histological changes, in a large epidemiological study design is required to elucidate the pathophysiological mechanism of unilaterally decreased renal growth after very preterm birth.
References
Ingelfinger JR (2004) Pathogenesis of perinatal programming. Curr Opin Nephrol Hypertens 13:459–464
Woods LL, Weeks DA, Rasch R (2004) Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int 65:1339–1348
Spencer J, Wang Z, Hoy W (2001) Low birth weight and reduced renal volume in Aboriginal children. Am J Kidney Dis 37:915–920
Konje JC, Okaro CI, Bell SC, de Chazal R, Taylor DJ (1997) A cross-sectional study of changes in fetal renal size with gestation in appropriate- and small-for-gestational-age fetuses. Ultrasound Obstet Gynecol 10:22–26
Singh GR, Hoy WE (2004) Kidney volume, blood pressure, and albuminuria: findings in an Australian Aboriginal community. Am J Kidney Dis 43:254–259
Hughson M, Farris AB, Douglas-Denton R, Hoy WE, Bertram JF (2003) Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 63:2113–2122
Brenner BM, Chertow GM (1993) Congenital oligonephropathy: an inborn cause of adult hypertension and progressive renal injury? Curr Opin Nephrol Hypertens 2:691–695
Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D (1991) Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64:777–784
Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE (2004) Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7:17–25
Rodriguez MM, Gomez A, Abitbol C, Chandar J, Montane B, Zilleruelo G (2005) Comparative renal histomorphometry: a case study of oligonephropathy of prematurity. Pediatr Nephrol 20:945–949
Schmidt IM, Chellakooty M, Boisen KA, Damgaard IN, Mau KC, Olgaard K, Main KM (2005) Impaired kidney growth in low-birth-weight children: distinct effects of maturity and weight for gestational age. Kidney Int 68:731–740
Drougia A, Giapros V, Hotoura E, Papadopoulou F, Argyropoulou M, Andronikou S (2009) The effects of gestational age and growth restriction on compensatory kidney growth. Nephrol Dial Transplant 24:142–148
Keijzer-Veen MG, Kleinveld HA, Lequin MH, Dekker FW, Nauta J, de Rijke YB, van der Heijden BJ (2007) Renal function and size at young adult age after intrauterine growth restriction and very premature birth. Am J Kidney Dis 50:542–551
Keijzer-Veen MG, Finken MJ, Nauta J, Dekker FW, Hille ET, Frolich M, Wit JM, van der Heijden AJ (2005) Is blood pressure increased 19 years after intrauterine growth restriction and preterm birth? A prospective follow-up study in The Netherlands. Pediatrics 116:725–731
Schmidt IM, Main KM, Damgaard IN, Mau C, Haavisto AM, Chellakooty M, Boisen KA, Petersen JH, Scheike T, Olgaard K (2004) Kidney growth in 717 healthy children aged 0–18 months: a longitudinal cohort study. Pediatr Nephrol 19:992–1003
Emamian SA, Nielsen MB, Pedersen JF, Ytte L (1993) Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR Am J Roentgenol 160:83–86
Battista MC, Oligny LL, St Louis J, Brochu M (2002) Intrauterine growth restriction in rats is associated with hypertension and renal dysfunction in adulthood. Am J Physiol Endocrinol Metabol 283:E124–E131
Keijzer-Veen MG, Schrevel M, Finken MJ, Dekker FW, Nauta J, Hille ET, Frolich M, van der Heijden BJ (2005) Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol 16:2762–2768
Bacchetta J, Harambat J, Dubourg L, Guy B, Liutkus A, Canterino I, Kassai B, Putet G, Cochat P (2009) Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int. doi:10.1038/ki.2009.201
Zumrutdal AO, Turan C, Cetin F, Adanali S (2002) Relationship between renal size and hypertension in patients with chronic renal failure. Nephron 90:145–147
Kennedy WA, Chitkara U, Abidari JM, Shortliffe LM (2003) Fetal renal growth as assessed through renal parenchymal area derived from prenatal and perinatal ultrasonography. J Urol 169:298–302
Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M (2003) A United States national reference for fetal growth. Obstet Gynecol 87:163–168
Schell-Feith EA, Que I, Kok DJ, Kist-Van Holthe JE, Kuhler E, Brand R, Papapoulos SE, van der Heijden BJ (2001) Modulation of calcium oxalate monohydrate crystallization kinetics by urine of preterm neonates. Am J Kidney Dis 38:1229–1234
Friberg P, Sundelin B, Bohman SO, Bobik A, Nilsson H, Wickman A, Gustafsson H, Petersen J, Adams MA (1994) Renin-angiotensin system in neonatal rats: induction of a renal abnormality in response to ACE inhibition or angiotensin II antagonism. Kidney Int 45:485–492
Tufro-McReddie A, Romano LM, Harris JM, Ferder L, Gomez RA (1995) Angiotensin II regulates nephrogenesis and renal vascular development. Am J Physiol 269:F110–F115
Ablett MJ, Coulthard A, Lee RE, Richardson DL, Bellas T, Owen JP, Keir MJ, Butler TJ (1995) How reliable are ultrasound measurements of renal length in adults? Br J Radiol 68:1087–1089
Emamian SA, Nielsen MB, Pedersen JF (1995) Intraobserver and interobserver variations in sonographic measurements of kidney size in adult volunteers. A comparison of linear measurements and volumetric estimates. Acta Radiol 36:399–401
Miletic D, Fuckar Z, Sustic A, Mozetic V, Stimac D, Zauhar G (1998) Sonographic measurement of absolute and relative renal length in adults. J Clin Ultrasound 26:185–189
Acknowledgements
This part of the POPS 19 study was supported by a grant of the Dutch Kidney Foundation. We thank TNO, Quality of Life (ETM Hille, P Verloove-Vanhorick) for the use of the POPS cohort database, MH Lequin for renal ultrasound measurements (Dept. Pediatric Radiology, Erasmus MC - Sophia, Rotterdam), and HA Kleinveld for her efforts in the data collection and analyses (Dept. Pediatric Nephrology, Erasmus MC - Sophia, Rotterdam).
Financial support: Dutch Kidney Foundation, Grant C-1924
No conflict of Interest
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Keijzer-Veen, M.G., Devos, A.S., Meradji, M. et al. Reduced renal length and volume 20 years after very preterm birth. Pediatr Nephrol 25, 499–507 (2010). https://doi.org/10.1007/s00467-009-1371-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-009-1371-y