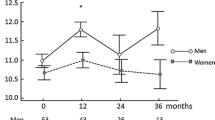
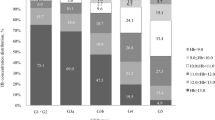
Abstract
Previous studies often report lower responses to erythropoietin (EPO) therapy in pediatric patients on chronic dialysis than those of adults. Because of the greater capacity for hematopoiesis in the younger population, these studies may be confounded by poorly identified variables. Thus, we made parallel studies of pediatric and adult cohorts to explore the relationship between age, gender and other risk factors with EPO resistance. Thirty pediatric subjects (aged 8–20 years) and 66 adult subjects (aged 22–85 years) on chronic hemodialysis and EPO were enrolled. After stratification by 50th percentile of EPO response, the best predictive model was identified by backward elimination of the risk factors with the least contribution to the regression. Relationship between age, gender and EPO resistance was examined by analysis of covariance (ANCOVA). The most predictive model of EPO response for the pediatric cohort had, as the major variables, urea clearance × dialysis duration/total body water (Kt/V), urea reduction ratio (URR), intact parathyroid hormone (iPTH), blood loss, normalized protein catabolic rates (nPCR) and indices of malnutrition and inflammation, whereas adults had iron and folate deficiencies as the dominant variables. Although EPO resistance was more common in female subjects than in male subjects, relationship with neither age nor gender was significant. Furthermore, the prescription of a larger (initiating) EPO dose by pediatric physicians compared with adult nephrologists confounded the interaction between age and EPO resistance. In summary EPO resistance in the pediatric dialysis cohort was predicted by nutritional deficits, inflammation, poor dialysis, and hyperparathyroidism, while iron and folate deficits were the major determinants in adults. Although confounded by the pattern of EPO prescription, neither age nor gender was predictive of EPO resistance in the two study groups.
Similar content being viewed by others
References
Eschbach JW (1989) The anemia of chronic renal failure: pathophysiology and the effects of recombinant erythropoietin. Kidney Int 35:134–148
Marsh J, Brown W, Wolcott D, Carr C, Harper R, Schweitzer S, Nissenson A (1991) rHuEPO treatment improves brain and cognitive function in anemic dialysis patients. Kidney Int 39:155–163
Revicki D, Brown R, Feeny D, Henry D, Teehan B, Rudnick M, Benz R (1991) Health related quality of life with human erythropoietin treatment for predialysis chronic renal disease patients. Am J Kidney Dis 25:548–554
Di Iorio B, Cirillo M, Bellizzi V, Stellato D, De Santo NG (2007) Prevalence and correlates of anemia and uncontrolled anemia in chronic hemodialysis patients: the Campania Dialysis Registry. Int J Artif Organs 30:325–333
Al-Hilali N, Al-Humoud H, Ninan VT, Nampoory MR, Puliyclil MA, Johny KV (2007) Does parathyroid hormone affect erythropoietin therapy in dialysis patients? Med Princ Pract 16:63–67
Seeherunvong W, Rubio L, Abitbol CL, Montane B, Strauss J, Diaz R, Zilleruelo G (2001) Identification of poor responders to erythropoietin among children undergoing hemodialysis. J Pediatr 138:710–714
Ifudu O, Uribarri J, Rajwani I, Vlacich V, Reydel K, Delosreyes G, Friedman EA (2000) Adequacy of dialysis and differences in hematocrit among dialysis facilities. Am J Kidney Dis 36:1166–1174
Varagunam M, McCloskey DJ, Sinnott PJ, Raftery MJ, Yaqoob MM (2003) Angiotensin-converting enzyme gene polymorphism and erythropoietin requirement. Perit Dial Int 23:111–115
Bamgbola OF, Kaskel F (2005) Role of folate deficiency on erythropoietin resistance in pediatric and adolescent patients on chronic dialysis. Pediatr Nephrol 20:1622–1629
Health Care Financing Administration (1998) 1998 Annual Report, End–Stage Renal Disease Core Indicators Project. Baltimore MD, Department of Health and Human Services, HCFA, Office of Clinical Standards and Quality
Frankfield DL, Rocco MV, Frederick PR, Pugh J, McClellan WM, Owen WF Jr (1991) Racial/ethnic analysis of elected intermediate outcomes for hemodialysis patients: results from the 1997 ESRD Core Indicators Project. Am J Kidney Dis 34:721–730
Ifudu O, Uribarri J, Rajwani I, Vlacich V, Reydel K, Delosreyes G, Friedman EA (2001) Gender modulates responsiveness to recombinant erythropoietin. Am J Kidney Dis 38:518–522
Zhang Y, Thamer M, Stefanik K, Kaufman J, Cotter DJ (2004) Epoietin requirements predict mortality in hemodialysis patients. Am J Kidney Dis 44:866–876
Van Damme-Lombaerts R, Broyer M, Businger J, Baldauf C, Stocker H (1994) A study of recombinant human erythropoietin in the treatment of anemia of chronic renal failure in children on hemodialysis. Pediatr Nephrol 8:338–342
Jabs K, Alexander S, McCabe D, Lerner G, Harmon WE (1994) Primary results from the US multicenter pediatric recombinant erythropoietin study. J Am Soc Nephrol 5:456–463
Palis J, Segel GB (1998) Developmental biology of erythropoiesis. Blood Rev 12:106–114
Collins AJ, Brenner RM, Ofman JJ, Chi EM, Stuccio-White N, Krishnan M, Solid C, Ofsthun NJ, Lazarus JM (2005) Epoetin alfa use in patients with ESRD: an analysis of recent US prescribing patterns and hemoglobin outcomes. Am J Kidney Dis 46:481–488
Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH (2001) A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38:1251–1263
El-Khatib M, Duncan HJ, Kant KS (2006) Role of C-reactive protein, reticulocyte hemoglobin content and inflammatory markers in iron and erythropoietin administration in dialysis patients. Nephrology 11:400–404
Lowrie EG, Chertow GM, Lew NL, Lazarus JM, Owen WF (1999) The urea [clearance x dialysis time] product (Kt) as an outcome-based measure of hemodialysis dose. Kidney Int 56:729–737
Cooper AC, Mikhail A, Lethbridge MW, Kemeny DM, Macdougall C (2003) Increased expression of erythropoiesis inhibiting cytokines (IFN-gamma, TNF-alpha, IL-10, and IL-13) by T cells in patients exhibiting a poor response to erythropoietin therapy. J Am Soc Nephrol 14:1776–1784
Yuen D, Richardson RM, Fenton SS, McGrath-Chong ME, Chan CT (2005) Quotidian nocturnal hemodialysis improves cytokine profile and enhances erythropoietin responsiveness. ASAIO J 51:236–241
Lin CL, Huang CC, Yu CC, Wu CH, Chang CT, Hsu PY, Yang CW (2002) Improved iron utilization and reduced erythropoietin resistance by on-line hemodiafiltration. Blood Purif 20:349–356
Gorman G, Furth S, Hwang W, Parekh R, Astor B, Fivush B, Frankenfield D, Neu A (2006) Clinical outcomes and dialysis adequacy in adolescent hemodialysis patients. Am J Kidney Dis 47:285–293
Chand DH, Brier M, Strife CF (2005) Comparison of vascular type in pediatric hemodialysis patients with respect to urea clearance, anemia management, and serum albumin concentration. Am J Kidney Dis 45:303–308
Roberts TL, Obrador GT, St Peter WL, Pereira BJ, Collins AJ (2004) Relationship among catheter insertions, vascular access infections, and anemia management in hemodialysis patients. Kidney Int 66:2429–2436
Movilli E, Brunori G, Camerini C, Vizzardi V, Gaggia P, Cassamali S, Scolari F, Parrinello G, Cancarini GC (2006) The kind of vascular access influences the baseline inflammatory status and epoietin response in chronic hemodialysis patients. Blood Purif 24:387–393
Sharples EJ, Varagunam M, Sinnott PJ, McCloskey DJ, Raftery MJ, Yaqoob MM (2006) The effect of proinflammatory cytokine gene and angiotensin-converting enzyme polymorphisms on erythropoietin requirements in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 16:64–68
Port RE, Kiepe D, Van Guilder M, Jellife RW, Mehls O (2004) Recombinant human erythropoietin for the treatment of renal anaemia in children: no justification for bodyweight-adjusted dosage. Clin Pharmacokinet 43:57–70
Besarab A (2006) Resolving the paradigm crisis in intravenous iron and erythropoietin management. Kidney Int Suppl 69:S13–S18
Chavers BM, Roberts TL, Herzog CA, Collins AJ, St Peter WL (2004) Prevalence of anemia in erythropoietin-treated pediatric as compared to adult chronic dialysis patients. Kidney Int 65:266–273
Kato A, Hamada M, Suzuki T, Maruyama Y, Hishida A (2001) Effect of weekly or successive iron supplementation on erythropoietin doses in patients receiving hemodialysis. Nephron 89:110–112
Axelsson J, Qureshi AR, Heimbϋger O, Lindholm B, Stenvikel P, Barany P (2005) Body fat mass and serum leptin levels influence epoietin sensitivity in patients with ESRD. Am J Kidney Dis 46:628–634
Nakamoto H, Kanno Y, Okada H, Suzuki H (2004) Erythropoietin resistance in patients on continuous ambulatory peritoneal dialysis. Adv Perit Dial 20:111–116
Odabas AR, Cetinkaya R, Selcuk Y, Keles S, Bilen H (2003) The effect of high dose losartan on erythropoietin resistance in patients undergoing hemodialysis. Panminerva Med 45:59–62
Schaefer F, Veldhuis JD, Robertson WR, Dunger D, Scharer K (1994) Immunoreactive and bioactive luteinizing hormone in pubertal patients with chronic renal failure. Cooperative Study Group on Pubertal Development in Chronic Renal Failure. Kidney Int 45:1465–1476
Di Iorio BR, Stellato D, De Santo NG, Cirillo M (2004) Association of gender and age with erythropoietin resistance in hemodialysis patients: role of menstrual status. Blood Purif 22:423–427
T’Sjoen GG, Beguin Y, Feyen E, Rubens R, Kaufman JM, Gooren L (2005) Influence of oestrogen or (anti-) androgen administration on soluble transferrin receptor in human plasma. J Endocrinol 186:61–67
Zeng SM, Yankowitz J, Widness JA, Strauss RG (2001) Etiology of differences in hematocrit between males and females: sequence-based polymorphisms in erythropoietin and its receptor. J Gend Specif Med 4:35–40
Hero M, Wickman S, Hanhijarvi R, Silmes MA, Dunkel L (2005) Pubertal upregulation of erythropoiesis in boys is determined primarily by androgen. J Pediatr 146:245–252
Le Petit-Thevenin J, Lerique B, Nobili O, Boyer J (1991) Estrogen modulates phospholipid acylation in red blood cells: relationship to cell aging. Am J Physiol 261:C423–C427
Paoletti E, Cannella G (2006) Update on erythropoietin treatment: should hemoglobin be normalized in patients with chronic kidney disease? J Am Soc Nephrol 17 [4 Suppl 2]:S74–S77
Acknowledgment
Supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Training Grant no. 9-526-3740
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Appendix A. Objective malnutrition inflammation scores
-
(a)
Serum albumin: (1) ≥ 4 g/dl = 0, (2) 3.5–3.9 g/dl = 1, (3) 3.0–3.4 g/dl = 2, (4) < 3.0 g/dl = 3
-
(b)
Serum transferrin: (1) ≥ 250 mg/dl = 3, (2) 200–249 mg/dl = 2, (3) 150–199 mg/dl = 1, (4) < 150 mg/dl = 0
-
(c)
Body mass index: (1) ≥ 20 kg/m2 = 0, (2) 15–19.9 kg/m2 = 1, (3) 16–17.9 kg/m2 = 2, (4) < 16 kg/m2 = 3
-
(d)
Dialysis duration (years): (1) 0–1 = 0, (2) 1–3 = 1, (3) 3–5 = 2, (4) > 5 = 3
-
(e)
Infection: (1) none = 0, (2) low grade (e.g. line-induced bacteremia, upper respiratory tract infection = 0.5 × no. of events, (3) moderate (e.g. symptomatic line sepsis, pneumonia) = 1.0 × no. of events, (4) severe (infection warranting hospitalization) = 1.5 × no. of events
-
(f)
Dialysis adequacy: (1) URR ≥ 80% = 0, (2) 61–79% = 1, (3) 41–60% = 2, (4) < 40% = 3
-
(g)
Serum ferritin: (1) 0–500 mg/dl = 0, (2) 501–750 mg/dl = 1, (3) 751–1,000 mg/dl = 2, (4) > 1,001 mg/dl = 3
Appendix B. Clinical and laboratory definitions
EPO resistance index
ERI calculated as EPO dose (international units per kilogram per week) divided by a given value of hemoglobin concentration (grams per deciliter).
Folate deficiency
Because of an overlap in the diagnostic accuracy of the red blood cell (RBC) parameters [e.g. mean corpuscular volume (MCV) and red cell distribution width (RDW) were poorly specific], folate deficiency (FD) was defined as (a) Mean serum folate and/or RBC folate ≤ 2 standard deviation score (b) Mean MCV ≥ 95th percentile for age and gender and/or RDW ≥ 16 and (c) Normal serum vitamin B12.
Iron deficiency
Fe deficiency consists of (a) Mean Fe saturation < 20% (b) Mean serum ferritin < 100 mg/dl and (c) Mean MCV [and/or mean corpuscular hemoglobin (MCH)] < 5th percentile for age and gender.
Malnutrition inflammatory score
The objective malnutrition inflammatory score (OMIS), adapted from the comprehensive MIS [18], is a questionnaire designed to quantify nutritional deficiency and inflammatory burdens. Its components are primary renal disease, dialysis duration, dialysis adequacy, C-reactive protein, total iron binding capacity, serum ferritin, body mass index, serum albumin and transferrin.
Dialysis adequacy
Urea reduction ratio (URR) = pre-dialysis blood urea nitrogen (BUN) − post-dialysis BUN/pre-dialysis BUN
Kt/V was determined by computer analysis (formal urea kinetic modeling) of the variables K, extrapolated from the mass transfer area coefficient (KoA) of the dialyzer, V, estimated total body water, and G, urea generation rate [or normalized protein catabolic rate (nPCR)].
Pubertal status
Post-puberty was defined as (a) Tanner stage (breast and/or pubic hair) ≥ 2, (b) plasma levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) consistent with the stage of puberty (c) and normal plasma free-thyroxine (FT4) and thyroid-stimulating hormone (TSH). Plasma gonadotropins were measured by ultra-sensitive chemiluminometric assay. Tanner I, FSH > 0.65 ng/ml, LH > 0.75 ng/ml; Tanner II, FSH > 2.75 ng/ml, LH > 1.0 ng/ml; Tanner III, FSH > 2.25 ng/ml, LH > 1.0 ng/ml; Tanner IV, FSH > 2.5 ng/ml, LH > 2.5 ng/ml; Tanner V, FSH > 2.5 ng/ml, LH > 2.5 ng/ml.
Infection score
Severity of infection: mild (e.g. line-induced bacteremia, upper respiratory tract infection) = 1, moderate (e.g. symptomatic line sepsis, pneumonia) = 2, and severe (infection warranting hospitalization) = 3.
Grade of surgery
minor (e.g. vascular access placement or revision) = 1, major (e.g. nephrectomy, parathyroidectomy, appendectomy) = 3
Estimated blood loss
Amount of blood collected for all laboratory procedures was measured; blood loss during dialysis (e.g. clotted circuit) was subjectively quantified by experienced dialysis nurses, and surgical blood loss was estimated by the attending surgeons. Blood loss was corrected for blood volume (BV) as EBL/BV. Calculated blood volume = 87 ml/kg for 7–18 years, and 72 ml/kg for > 18 years.
Residual renal function
An estimated minimum daily urine output of 50 ml and 100 ml for study subjects below 10 years of age and above 10 years of age, respectively.
Rights and permissions
About this article
Cite this article
Bamgbola, O.F., Kaskel, F.J. & Coco, M. Analyses of age, gender and other risk factors of erythropoietin resistance in pediatric and adult dialysis cohorts. Pediatr Nephrol 24, 571–579 (2009). https://doi.org/10.1007/s00467-008-0954-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0954-3