Abstract
Background
Transanal total mesorectal excision (TaTME) appears to have favorable surgical and pathological outcomes. However, the evidence on survival outcomes remains unclear. We performed a meta-analysis to compare long-term oncologic outcomes of TaTME with transabdominal TME for rectal cancer.
Methods
PubMed, EMBASE, and the Cochrane Library were searched. Data were pooled, and overall effect size was calculated using random-effects models. Outcome measures were overall survival (OS), disease-free survival (DFS), and local and distant recurrence.
Results
We included 11 nonrandomized studies that examined 2,143 patients for the meta-analysis. There were no significant differences between the two groups in OS, DFS, and local and distant recurrence with a RR of 0.65 (95% CI 0.39–1.09, I2 = 0%), 0.79 (95% CI 0.57–1.10, I2 = 0%), 1.14 (95% CI 0.44–2.91, I2 = 66%), and 0.75 (95% CI 0.40–1.41, I2 = 0%), respectively.
Conclusion
In terms of long-term oncologic outcomes, TaTME may be an alternative to transabdominal TME in patients with rectal cancer. Well-designed randomized trials are warranted to further verify these results.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Total mesorectal excision (TME) has been considered the standard surgical procedure for patients with rectal cancer since it was first described in 1982 by Heald [1]. This procedure was initially performed with an open abdominal approach, and laparoscopic TME has been recently suggested as an alternative to open TME [2,3,4]. However, the surgical technique is complex and requires extensive experience to safely perform for high-quality surgical resection and good oncologic outcomes, particularly in patients with lower rectal cancer. With recent advances in minimally invasive surgery, a transanal and laparoscopic combined approach was introduced as transanal TME (TaTME), and this was proposed as a possibility for overcoming the technical difficulties of transabdominal TME [5]. Although a majority of rectal cancers can be safely operated on with the transabdominal approach, difficult anatomical conditions, unfavorable tumor characteristics, or a combination of these factors can lead to difficulties. Narrow pelvis, fatty mesorectum, male sex, high BMI, and anterior-located large tumor are risk factors for noncurative resection [6]. The transanal approach may provide better access and visualization of the distal part of the rectum.
Many studies, including a meta-analysis, have reported favorable results in terms of perioperative, pathological, and functional outcomes in patients receiving TaTME for rectal cancer. The above-mentioned risk factors, in combination with the difficulty of perpendicular division of the rectum, seem to be related to circumferential resection margin (CRM) involvement, incompleteness of TME, and anastomotic leakage, which are considered to have negative oncologic impacts [7,8,9,10,11,12,13]. However, despite favorable results for CRM involvement, incompleteness of TME, and anastomotic leakage in TaTME, there is still a lack of evidence on long-term oncologic outcomes to support its widespread introduction. Therefore, our aim was to conduct a systematic review and meta-analysis to evaluate survival outcomes such as 2-year or 3-year survivals, or if possible 5-year survivals, and recurrence rates of TaTME in comparison with transabdominal TME in patients with rectal cancer. Evaluated outcomes were overall survival (OS), disease-free survival (DFS), and local and distant recurrence.
Methods
This meta-analysis followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14]. Multiple comprehensive databases were searched for studies that assessed the long-term oncologic outcomes of TaTME compared with transabdominal TME for rectal cancer. The study protocol used Cochrane Review Methods [15]. IRB approval was not needed for this article.
Data and literature sources
Studies were identified from PubMed (January 1, 1976 to April 7, 2020), EMBASE (January 1, 1985 to April 7, 2020), and the Cochrane Central Register of Controlled Trials (CENTRAL) (January 1, 1987 to April 7, 2020). There were no restrictions regarding the year of publication, and articles in any language were permitted for review. The search terms were "rectal cancer," "transanal TME," "recurrence," "prognosis," and "survival." After the preliminary electronic search, further articles were searched for manually to retrieve additional studies. Finally, all articles were assessed individually for inclusion.
Study selection and data extraction
Article titles and abstracts were screened and full texts were independently reviewed by two reviewers (JY Moon and GW Ha) according to the selection criteria. Any differences in judgment regarding inclusion were resolved through discussion between the reviewers.
The included studies assessed survival outcomes, including OS, DFS, local recurrence, and distant recurrence, in patients with rectal cancer who were treated with TaTME or transabdominal TME. All of the surgical modalities such as open, laparoscopic, and robotic surgery were included in both TME approaches if possible. Studies were excluded if they (i) did not compare TaTME with transabdominal TME; (ii) assessed patients with stage IV or recurred rectal cancer; (iii) assessed only patients who received abdominoperineal resection; (iv) had no extractable data and authors were unavailable to provide additional information; or (v) were case series with fewer than 10 patients.
All eligible studies were reviewed and all relevant data were extracted by the two reviewers independently using a data extraction form designed before the review. The variables recorded were (i) standard publication information, including year of publication, name of the first author, and number of patients; (ii) clinical and demographic characteristics of included studies; and (iii) outcomes (OS, DFS, local recurrence, and distant recurrence).
Assessment of methodological quality
The methodological quality of the studies included in the meta-analysis was assessed using the Newcastle–Ottawa quality scale (NOS), which attributes a maximum of 9 points to each study and categorizes a study with a score of 6 or more as “high quality” [16]. The quality of the included studies was analyzed using 3 categories: patient selection, comparability, and outcome assessment.
Statistical analysis
For dichotomous outcomes, relative risk (RR), variance, and 95% confidence interval (CI) were determined in the meta-analysis. The presence and amount of heterogeneity were assessed using the Q test and I2 index, respectively; a p-value less than 0.1 was considered statistically significant [17]. The DerSimonian-Laird random-effects model (REM) was used to pool data in light of cross-study heterogeneity [18].
First, we performed a meta-analysis to evaluate survival outcomes such as OS, DFS, and local and distant recurrence of TaTME in comparison with transabdominal TME in patients with rectal cancer. Second, we performed a meta-analysis to compare CRM involvement, incompleteness of TME, and anastomotic leakage between the two groups. Sensitivity analyses were performed to assess the robustness of the meta-analysis findings [19, 20]. First, studies with a higher rate of CRM involvement in the transabdominal TME group than in the TaTME group were analyzed. Second, studies with a higher rate of incomplete TME in the transabdominal TME group than in the TaTME group were analyzed. Third, studies with a higher rate of anastomotic leakage in the transabdominal TME group than in the TaTME group were analyzed. Fourth, studies with large outlying effects or studies with a score less than 6 in the NOS scale, indicating low quality, were excluded. Fifth, the trim-and-fill method and analysis with an alternative effect size were performed.
Funnel plots were used to determine the presence of publication bias by visual inspection of funnel plots and the Egger-weighted linear regression test; a p-value less than 0.1 was considered statistically significant [21, 22]. Data analyses were performed using Review Manager software (version 5.4) from the Cochrane Collaboration and Comprehensive Meta-Analysis software (version 3).
Results
Description of studies
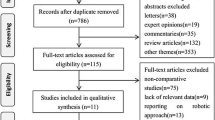
The predefined search strategy identified 1,831 potentially relevant articles. We excluded 451 articles because they were duplicates and 1,365 articles because their titles and abstracts did not fulfill the selection criteria. After full text review of the remaining 15 articles, we excluded 4 articles because of the exclusion criteria of this study. Therefore, we included 11 nonrandomized studies that examined 2,143 patients for qualitative analysis and meta-analysis (Fig. 1). Among included patients, 529 patients received TaTME. Six studies evaluated OS and DFS [23,24,25,26,27,28], 11 studies evaluated local recurrence [23,24,25,26,27,28,29,30,31,32,33], and five studies evaluated distant recurrence [23, 26, 30,31,32]. Most of the included studies evaluated patients who underwent laparoscopic TaTME, while one study evaluated patients who underwent open TaTME [24]. Most of the included studies evaluated patients who underwent transabdominal TME with the laparoscopic approach only; two studies included patients who underwent transabdominal TME with laparoscopic or open approaches [24, 33], and one study included patients who underwent transabdominal TME with a robotic TME approach [30]. Evaluation of methodological quality showed that all studies scored at least 6 points (≥6) on the NOS scale. Tables 1 and 2 summarize the characteristics of the included studies.
Long-term oncologic outcomes of TaTME compared with transabdominal TME
Analysis of oncologic outcomes for TaTME in patients with rectal cancer indicated that 6 studies (604 patients) reported data on OS; there were no significant survival differences between TaTME and transabdominal TME (risk ratio [RR] = 0.65, 95% confidence interval [CI] = 0.39–1.09, I2 = 0%) (Fig. 2). Six studies (604 patients) reported data on DFS; there were no significant survival differences between the two groups (RR = 0.79, 95% CI = 0.57–1.10, I2 = 0%) (Fig. 3). Eleven studies (2,143 patients) reported data on local recurrence; there were no significant differences between two groups (RR = 1.14, 95% CI = 0.44–2.91, I2 = 66%) (Fig. 4). Five studies (329 patients) reported data on distant recurrence; there were no significant differences between two groups (RR = 0.75, 95% CI = 0.40–1.41, I2 = 0%) (Fig. 5). Sensitivity analyses using predefined methods indicated that the results of these meta-analyses were robust.
Analyses of CRM involvement, incompleteness of TME, and anastomotic leakage
Comparing CRM involvement between the two groups, the TaTME group was associated with better outcomes, with a RR of 0.44 (95% CI 0.27–0.87, I2 = 0%) (Fig. 6a). Analysis to compare incompleteness of TME showed no significant differences between TaTME and transabdominal TME groups, with a RR of 0.88 (95% CI 0.50–1.55, I2 = 0%) (Fig. 6b). Analysis to compare anastomotic leakage also showed no significant differences between TaTME and transabdominal TME groups, with a RR of 0.94 (95% CI 0.58–1.54, I2 = 27%) (Fig. 6c).
Analysis of oncologic outcomes according to rate of CRM involvement
Analysis of studies with a higher rate of CRM involvement in the transabdominal TME group than in the TaTME group showed no significant differences between the two groups in analysis of OS, DFS, local recurrence, and distant recurrence with a RR of 0.65 (95% CI 0.39–1.09, I2 = 0%), 0.79 (95% CI 0.57–1.10, I2 = 0%), 0.72 (95% CI 0.39–1.36, I2 = 0%), and 0.75 (95% CI 0.40–1.41, I2 = 0%), respectively (Fig. 7).
Analysis of oncologic outcomes according to rate of TME incompleteness
Analysis of studies with a higher rate of incomplete TME in the transabdominal TME group than in the TaTME group showed no significant differences between the two groups in analysis of OS, DFS, local recurrence, and distant recurrence with a RR of 0.67 (95% CI 0.39–1.14, I2 = 0%), 0.71 (95% CI 0.48–1.05, I2 = 0%), 0.57 (95% CI 0.25–1.33, I2 = 0%), and 0.59 (95% CI 0.25–1.39, I2 = 0%), respectively (Fig. 7).
Analysis of oncologic outcomes according to rate of anastomotic leakage
Analysis of studies with a higher rate of anastomotic leakage in the transabdominal TME group than in the TaTME group showed no significant differences between the two groups in analysis of OS, DFS, local recurrence, and distant recurrence with a RR of 0.67 (95% CI 0.39–1.14, I2 = 0%), 0.71 (95% CI 0.48–1.05, I2 = 0%), 0.65 (95% CI 0.29–1.45, I2 = 0%), and 0.74 (95% CI 0.39–1.42, I2 = 0%), respectively (Fig. 7).
Publication bias
Publication bias was determined by visual inspection of funnel plots and the Egger-weighted linear regression test to assess any asymmetry in the funnel plots. The results showed that the funnel plots for local recurrence (p = 0.045) were asymmetrical, indicating a presence of publication bias.
Discussion
To our knowledge, despite a relatively small number of included patients, this study is the first meta-analysis to compare long-term oncologic outcomes between TaTME and transabdominal TME. Since TaTME was introduced in 2010 [5], many studies have reported favorable perioperative, pathological, and functional outcomes, although little is known about the long-term oncologic outcomes of TaTME such as OS, DFS, and distant recurrence. Our findings on the long-term oncologic outcomes of TaTME may illustrate its oncologic safety and support its introduction and application.
Our meta-analysis showed no significant difference between TaTME and transabdominal TME in OS, DFS, local recurrence, and distant recurrence. The TaTME group had favorable CRM involvement compared with the transabdominal TME group. However, despite tendencies for lower rates of incompleteness of TME and anastomotic leakage in the TaTME group, there was no significant difference between the two groups in terms of incompleteness of TME and anastomotic leakage. Based on previous meta-analyses [11, 13], we considered lower rates of CRM involvement, incompleteness of TME, and anastomotic leakage in the TaTME group could demonstrate adequately performed TaTME procedures, which might show survival outcomes properly after overcoming the initial learning curve. Thus, we performed sensitivity analyses using predefined methods, such as analyses of long-term oncologic outcomes related to CRM involvement, incompleteness of TME, and anastomotic leakage, which indicated no statistical significance, suggesting the robustness of these results.
Studies have shown that CRM is an accepted surrogate marker for local recurrence and those with involved CRM have an increased risk of local recurrence [34, 35]. However, in our study, although the TaTME group had favorable CRM involvement and most included studies reported a higher rate of CRM involvement in the transabdominal TME group [23,24,25,26,27,28,29,30,31,32], margin involvement does not translate into significant differences in the rates of OS, DFS, distant recurrence, and local recurrence between the two groups. Another surrogate marker for local recurrence is the quality of the mesorectum [36]. In our study, analysis of incompleteness of TME showed no significance, and analysis of studies that reported a higher rate of incomplete mesorectum in the transabdominal TME group [23,24,25,26, 29] showed no significance in the rates of OS, DFS, distant recurrence, and local recurrence between the two groups. Anastomotic leakage may also have a negative effect on recurrence and survival outcomes [37,38,39]. In our study, analysis of anastomotic leakage showed no significance, and analysis of studies that reported a higher rate of anastomotic leakage in the transabdominal TME group [23,24,25,26, 30, 31] showed no significance in the rates of OS, DFS, distant recurrence, and local recurrence between the two groups. However, it is important to point out the relatively small number of included patients and the trends for better survival outcomes in TaTME group. The transanal approach with advances in technique and quality control will provide more patient data for analysis of the oncologic impact of TaTME. Consequently, as patient data increases, less CRM involvement, less TME incompleteness, and less anastomotic leakage may have a significantly positive effect on TaTME survival outcomes and recurrence.
Recently, TaTME for rectal cancer was suspended in Norway due to an unexpected higher recurrence rate after TaTME [40]. In our meta-analysis, except for one study [33], all included studies reported an acceptable local recurrence rate. After excluding this study, the result of local recurrence analysis had a trend for better outcomes in the TaTME group. One explanation may involve the technical aspect of rectal transection and air flow during dissection from the perineum, which could potentially allow the spread of tumor cells into the pelvic cavity [41]. Therefore, to ensure complete occlusion of the rectal lumen and reduce the possibility of tumor cells spreading, a modification of the technique to reinforce the purse-string has been proposed [42]. Before full-thickness incision of the rectum, placing a gauze swab in the lumen can also prevent tumor cell spillage [26].
There are some limitations to this study that make it difficult to draw strong conclusions. One limitation of this study is it lacks large randomized trials, and that the majority of the studies are retrospective and have a small number of patients. Second, there may be a potential heterogeneity among the included studies, even though we performed a sensitivity analysis. Clinical characteristics of patients may be various because comparative studies without randomization were included. Moreover, the procedures were performed by many different surgeons, and any non-standardized techniques used may have influenced the oncologic outcomes. Although TaTME is usually recommended as dissection of the distal one-third of the mesorectum [43], the level of rectal dissection via TaTME may vary between patients. Third, there are variations in the follow-up period among the included studies, and this might have affected the results.
In conclusion, although it remains in a stage of development, TaTME may offer favorable long-term oncologic outcomes and be an alternative to transabdominal TME in patients with distal rectal cancer. Well-designed large randomized trials are warranted to provide more definitive survival results.
References
Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1:1479–1482
Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, Chang HJ, Lee HS, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim DH, Oh JH (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645
van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218
Sun Z, Kim J, Adam MA, Nussbaum DP, Speicher PJ, Mantyh CR, Migaly J (2016) Minimally invasive versus open low anterior resection: equivalent survival in a national analysis of 14,033 patients with rectal cancer. Ann Surg 263:1152–1158
Sylla P, Rattner DW, Delgado S, Lacy AM (2010) NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 24:1205–1210
Rouanet P, Mourregot A, Azar CC, Carrere S, Gutowski M, Quenet F, Saint-Aubert B, Colombo PE (2013) Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum 56:408–415
Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP (2017) Transanal total mesorectal excision: international registry results of the first 720 cases. Ann Surg 266:111–117
Denost Q, Adam JP, Rullier A, Buscail E, Laurent C, Rullier E (2014) Perineal transanal approach: a new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg 260:993–999
Tuech JJ, Karoui M, Lelong B, De Chaisemartin C, Bridoux V, Manceau G, Delpero JR, Hanoun L, Michot F (2015) A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg 261:228–233
de Lacy FB, van Laarhoven J, Pena R, Arroyave MC, Bravo R, Cuatrecasas M, Lacy AM (2018) Transanal total mesorectal excision: pathological results of 186 patients with mid and low rectal cancer. Surg Endosc 32:2442–2447
Aubert M, Mege D, Panis Y (2020) Total mesorectal excision for low and middle rectal cancer: laparoscopic versus transanal approach-a meta-analysis. Surg Endosc 34:3908–3919
van der Heijden JAG, Koeter T, Smits LJH, Sietses C, Tuynman JB, Maaskant-Braat AJG, Klarenbeek BR, de Wilt JHW (2020) Functional complaints and quality of life after transanal total mesorectal excision: a meta-analysis. Br J Surg 107:489–498
Zhang X, Gao Y, Dai XL, Zhang HT, Shang ZJ, Cai XY, Shen T, Cheng XS, Yu K, Li YF (2019) Short- and long-term outcomes of transanal versus laparoscopic total mesorectal excision for mid-to-low rectal cancer: a meta-analysis. Surg Endosc 33:972–985
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269
Higgins JP, Green S (2011) Cochrane handbook for systematic reviews of interventions. Version 5.1.0, vol 5. Wiley, Chichester
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 May 2020
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Thabane L, Akhtar-Danesh N (2008) Guidelines for reporting descriptive statistics in health research. Nurse Res 15:72–81
Patsopoulos NA, Evangelou E, Ioannidis JP (2008) Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 37:1148–1157
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR (2000) Empirical assessment of effect of publication bias on meta-analyses. BMJ 320:1574–1577
de’angelis N, Portigliotti L, Azoulay D, Brunetti F (2015) Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbeck’s Arch Surg 400:945–959
Xu C, Song H-Y, Han S-L, Ni S-C, Zhang H-X, Xing C-G (2017) Simple instruments facilitating achievement of transanal total mesorectal excision in male patients. World J Gastroenterol 23:5798–5808
Lelong B, Meillat H, Zemmour C, Poizat F, Ewald J, Mege D, Lelong JC, Delpero JR, de Chaisemartin C (2017) Short- and mid-term outcomes after endoscopic transanal or laparoscopic transabdominal total mesorectal excision for low rectal cancer: a single institutional case-control study. J Am Coll Surg 224:917–925
Denost Q, Loughlin P, Chevalier R, Celerier B, Didailler R, Rullier E (2018) Transanal versus abdominal low rectal dissection for rectal cancer: long-term results of the Bordeaux’ randomized trial. Surg Endosc 32:1486–1494
Chen Y-T, Kiu K-T, Yen M-H, Chang T-C (2019) Comparison of the short-term outcomes in lower rectal cancer using three different surgical techniques: transanal total mesorectal excision (TME), laparoscopic TME, and open TME. Asian J Surg 42:674–680
Chen P, Yang S (2019) Conventional laparoscopy versus transanal total mesorectal excision(tatme) for rectal cancer after neoadjuvant chemoradiation: long-term follow-up results. Dis Colon Rect 62:e191
Marks JH, Montenegro GA, Salem JF, Shields MV, Marks GJ (2016) Transanal TATA/TME: a case-matched study of taTME versus laparoscopic TME surgery for rectal cancer. Tech Coloproctol 20:467–473
Lee KY, Shin JK, Park YA, Yun SH, Huh JW, Cho YB, Kim HC, Lee WY (2018) Transanal endoscopic and transabdominal robotic total mesorectal excision for mid-To-low rectal cancer: comparison of short-Term postoperative and oncologic outcomes by using a case-matched analysis. Ann Coloproctol 34:29–35
Mege D, Hain E, Lakkis Z, Maggiori L, la DenisePanis PÀY (2018) Is trans-anal total mesorectal excision really safe and better than laparoscopic total mesorectal excision with a perineal approach first in patients with low rectal cancer? A learning curve with case-matched study in 68 patients. Colorectal Dis 20:O143–O151
Gordeyev SS, Dzhumabaev KE, Mamedli ZZ, Kozlov NA, Surayeva YE, Fedyanin MY, Rasulov AO (2019) Transanal total mesorectal excision in selected patients with “difficult pelvis”: a case–control study of “difficult” rectal cancer patients. Eur Surg Acta Chirurgica Austriaca 51:13–18
Wasmuth HH, Færden AE, Myklebust TÅ, Pfeffer F, Norderval S, Riis R, Olsen OC, Lambrecht JR, Kørner H, Larsen SG, Forsmo HM, Bækkelund O, Lavik S, Knapp JC, Sjo O, Rashid G (2020) Transanal total mesorectal excision for rectal cancer has been suspended in Norway. Br J Surg 107:121–130
Quirke P, Durdey P, Dixon MF, Williams NS (1986) Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 2:996–999
Birbeck KF, Macklin CP, Tiffin NJ, Parsons W, Dixon MF, Mapstone NP, Abbott CR, Scott N, Finan PJ, Johnston D, Quirke P (2002) Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg 235:449–457
Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O’Callaghan C, Myint AS, Bessell E, Thompson LC, Parmar M, Stephens RJ, Sebag-Montefiore D (2009) Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 373:821–828
Ptok H, Marusch F, Meyer F, Schubert D, Gastinger I, Lippert H (2007) Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br J Surg 94:1548–1554
Kulu Y, Tarantio I, Warschkow R, Kny S, Schneider M, Schmied BM, Büchler MW, Ulrich A (2015) Anastomotic leakage is associated with impaired overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg Oncol 22:2059–2067
Ha GW, Kim JH, Lee MR (2017) Oncologic impact of anastomotic leakage following colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg Oncol 24:3289–3299
Larsen SG, Pfeffer F, Kørner H, Group NCC (2019) Norwegian moratorium on transanal total mesorectal excision. Br J Surg 106:1120–1121
Peacock O, Chang GJ (2020) Is the learning curve the Achilles heel of surgical innovation? —arguments against TaTME. Ann Laparosc Endosc Surg 5:45
Koch MJ, Tanis PJ, Bemelman WA, Tuynman JB, Hompes R, Belgers HJ (2020) Purse-string reinforcement in transanal total mesorectal excision: a further essential step to increase oncological safety - a video vignette. Colorectal Dis 22:219–220
Deijen CL, Velthuis S, Tsai A, Mavroveli S, de Lange-de Klerk ES, Sietses C, Tuynman JB, Lacy AM, Hanna GB, Bonjer HJ (2016) COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc 30:3210–3215
Acknowledgements
This paper was supported by the Fund of Biomedical Research Institute, Jeonbuk National University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jae Young Moon, Min Ro Lee, and Gi Won Ha have declared that no potential conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was supported by Fund of Biomedical Research Institute, Jeonbuk National University Hospital (CUH2020-0038)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moon, J.Y., Lee, M.R. & Ha, G.W. Long-term oncologic outcomes of transanal TME compared with transabdominal TME for rectal cancer: a systematic review and meta-analysis. Surg Endosc 36, 3122–3135 (2022). https://doi.org/10.1007/s00464-021-08615-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08615-7