Abstract
Background
Localization of colorectal tumors during laparoscopic surgery is generally performed by tattooing into the submucosal layer of the colon. However, faint and diffuse tattoos may lead to difficulties in recognizing cancer sites, resulting in inappropriate resection of the colon. We previously demonstrated that yttrium oxide nanoparticles doped with the rare earth ions (ytterbium and erbium) (YNP) showed strong near-infrared (NIR) emission under NIR excitation (1550 nm emission with 980 nm excitation). NIR light can penetrate deep tissues. In this study, we developed an NIR laparoscopy imaging system and demonstrated its use for accurate resection of the colon in swine.
Methods
The NIR laparoscopy system consisted of an NIR laparoscope, NIR excitation laser diode, and an NIR camera. Endo-clips coated with YNP (NIR clip), silicon rubber including YNP (NIR silicon mass), and YNP solution (NIR ink) were prepared as test NIR markers. We used a swine model to detect an assumed colon cancer site using NIR laparoscopy, followed by laparoscopic resection. The NIR markers were fixed at an assumed cancer site within the colon by endoscopy. An NIR laparoscope was then introduced into the abdominal cavity through a laparoscopy port.
Results
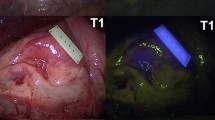
NIR emission from the markers in the swine colon was successfully recognized using the NIR laparoscopy imaging system. The position of the markers in the colon could be identified. Accurate resection of the colon was performed successfully by laparoscopic surgery under NIR fluorescence guidance. The presence of the NIR markers within the extirpated colon was confirmed, indicating resection of the appropriate site.
Conclusions
NIR laparoscopic surgery is useful for colorectal cancer site recognition and accurate resection using laparoscopic surgery.




Similar content being viewed by others
References
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AMH, Heath RM, Brown JM, Grp MCT (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Group COoSTS (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059
Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, Visa J (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 359:2224–2229
Lane KL, Vallera R, Washington K, Gottfried MR (1996) Endoscopic tattoo agents in the colon. Tissue responses and clinical implications. Am J Surg Pathol 20:1266–1270
Arteaga-Gonzalez I, Martin-Malagon A, Fernandez EM, Arranz-Duran J, Parra-Blanco A, Nicolas-Perez D, Quintero-Carrion E, Luis HD, Carrillo-Pallares A (2006) The use of preoperative endoscopic tattooing in laparoscopic colorectal cancer surgery for endoscopically advanced tumors: a prospective comparative clinical study. World J Surg 30:605–611
Trakarnsanga A, Akaraviputh T (2011) Endoscopic tattooing of colorectal lesions: Is it a risk-free procedure? World J Gastrointest Endosc 3:256–260
Zako T, Hyodo H, Tsuji K, Tokuzen K, Kishimoto H, Ito M, Kaneko K, Maeda M, Soga K (2010) Development of near infrared-fluorescent nanophosphors and applications for cancer diagnosis and therapy. J Nanomater 2010:491471
Hemmer E, Venkatachalam N, Hyodo H, Hattori A, Ebina Y, Kishimoto H, Soga K (2013) Upconverting and NIR emitting rare earth based nanostructures for NIR-bioimaging. Nanoscale 5:11339–11361
Welsher K, Liu Z, Sherlock SP, Robinson JT, Chen Z, Daranciang D, Dai H (2009) A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat Nanotechnol 4:773–780
Escobedo JO, Rusin O, Lim S, Strongin RM (2010) NIR dyes for bioimaging applications. Curr Opin Chem Biol 14:64–70
Smith AM, Mancini MC, Nie S (2009) Bioimaging: second window for in vivo imaging. Nat Nanotechnol 4:710–711
Yuan L, Lin W, Zheng K, He L, Huang W (2013) Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem Soc Rev 42:622–661
Soga K, Tokuzen K, Fukuda K, Hyodo H, Hemmer E, Venkatachalm N, Kishimoto H (2012) Application of ceramic/polymer conjugate materials for near infrared biophotonics. J Photopolym Sci Technol 25:57–62
Kamimura M, Kanayama N, Tokuzen K, Soga K, Nagasaki Y (2011) Near-infrared (1550 nm) in vivo bioimaging based on rare-earth doped ceramic nanophosphors modified with PEG-b-poly(4-vinylbenzylphosphonate). Nanoscale 3:3705–3713
Venkatachalam N, Yamano T, Hemmer E, Hyodo H, Kishimoto H, Soga K (2013) Er3+-doped Y2O3 nanophosphors for near-infrared fluorescence bioimaging applications. J Am Ceram Soc 96:2759–2765
Zako T, Yoshimoto M, Hyodo H, Kishimoto H, Ito M, Kaneko K, Soga K, Maeda M (2015) Cancer-targeted near infrared imaging using rare earth ion-doped ceramic nanoparticles. Biomater Sci 3:59–64
Venkatachalam N, Saito Y, Soga K (2009) Synthesis of Er3+ doped Y2O3 nanophosphors. J Am Ceram Soc 92:1006–1010
Raju GS, Gajula L (2004) Endoclips for GI endoscopy. Gastrointest Endosc 59:267–279
Kamimura M, Miyamoto D, Saito Y, Soga K, Nagasaki Y (2008) Design of poly(ethylene glycol)/streptavidin coimmobilized upconversion nanophosphors and their application to fluorescence biolabeling. Langmuir 24:8864–8870
Watanabe M, Takemura, H, Mizoguchi, H, Hyodo, H, Soga, K, Zako, T, Kishimoto, H, Ito, M, Kaneko, K (2013) Development of Novel Endoscope with NIR camera using real-time composite method. In: Proceedings of the 15th international conference on biomedical engineering, pp 128–131
Horn BKP (1986) Robot vision. MIT press, Cambridge
Palmer RJ, Butenhoff JL, Stevens JB (1987) Cytotoxicity of the rare earth metals cerium, lanthanum, and neodymium in vitro: comparisons with cadmium in a pulmonary macrophage primary culture system. Environ Res 43:142–156
Schubert D, Dargusch R, Raitano J, Chan SW (2006) Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun 342:86–91
Acknowledgments
We are grateful to Machida Endoscope Co. and Mr. Go Mita (Mitagiken Co. Ltd) for custom-made laparoscopy and NIR-VIS image splitter system, respectively. We thank Dr. Kenichi Koushi, MD (Department of Colorectal Surgery, National Cancer Center Hospital East) for tattooing images in laparoscopic surgery, and Prof. Toshiyuki Watanabe and Ms. Shiori Abe (Nagoya Zokei University) for the (Fig. 1) illustrations. We also thank doctors from National Cancer Center Hospital East and students from Tokyo University of Science (Soga laboratory and Kishimoto laboratory) for the help in laparoscopic surgery using model swine.
Funding
New Energy and Industrial Technology Development Organization (NEDO) of Japan (Industrial Technology Research Grant Program in 2009), RIKEN (Molecular Systems research), National Cancer Center Research & Development Fund (25-A-8 & 26-A-17) and Accelerating Regulatory Science Initiative (H-24), Program for Development of Strategic Research Center in Private Universities supported by Ministry of Education, Culture, Sport, Science, and Technology, Japan (MEXT), and Tokyo University of Science, 2009–2013 (S0901020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Tamotsu Zako, Masaaki Ito, Hiroshi Hyodo, Miya Yoshimoto, Masayuki Watanabe, Hiroshi Takemura, Hidehiro Kishimoto, Kazuhiro Kaneko, Kohei Soga and Mizuo Maeda have no conflict of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Zako, T., Ito, M., Hyodo, H. et al. Extra-luminal detection of assumed colonic tumor site by near-infrared laparoscopy. Surg Endosc 30, 4153–4159 (2016). https://doi.org/10.1007/s00464-015-4669-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4669-9