Abstract
Background
Epidermal growth factor (EGF) stimulates tumor growth directly via tumor cell EGF receptors or indirectly via its proangiogenic effects. This study’s purpose was to determine the impact of minimally invasive colorectal resection (MICR) on postoperative (postop) plasma EGF levels in the colorectal cancer (CRC) and benign disease settings and to see if preoperative (PreOp) EGF levels are altered in cancer patients.
Methods
MICR patients with benign pathology (n = 40) and CRC (n = 48) had blood samples taken PreOp and on postoperative days (POD) 1 and 3. In some patients, late samples were taken between POD7 and POD60; these were bundled into 7-day blocks and considered as single time points. EGF levels were determined by enzyme-linked immunosorbent assay (ELISA) and results were reported as mean ± SD after logarithmic transformation. The Student t test was used (p < 0.008 after Bonferroni correction).
Results
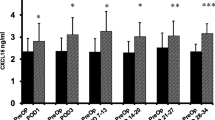
The cancer and benign groups were comparable except for age. The mean PreOp CRC plasma EGF level (122.9 ± 75.9 pg/ml) was significantly higher than that of the benign group (85.3 ± 38.5 pg/ml) (p = 0.015). The cancer group’s EGF levels were significantly decreased on POD1 and POD3 and for the POD31-55 time point (mean EGF level = 63.1 ± 42.2 (n = 10). The benign group’s POD3 and POD7-14 EGF levels were significantly lower than the PreOp level; later levels returned toward baseline. Small late sample size limited analysis.
Conclusion
Plasma EGF levels are significantly higher in cancer patients. MICR is associated with a significant decrease in EGF levels early postop in both cancer and benign settings. Unlike the benign group, EGF blood levels in cancer patients remain low during the second postop month. A larger study with more late samples is needed to verify these results. EGF may have value as a tumor marker.


Similar content being viewed by others
References
Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL (1998) Increased tumor establishment and growth after open vs laparoscopic bowel resection in mice. Surg Endosc 12:1035–1038
Carter JJ, Feingold DL, Kirman I, Oh A, Wildbrett P, Asi Z, Fowler R, Huang E, Whelan RL (2003) Laparoscopic-assisted cecectomy is associated with decreased formation of postoperative pulmonary metastases compared with open cecectomy in a murine model. Surgery 134:432–436
Weese JL, Ottery FD, Emoto SE (1986) Do operations facilitate tumour growth? An experimental model in rats. Surgery 100:273–277
Lennard TW, Shenton BK, Borzotta A, Donnelly PK, White M, Gerrie LM, Proud G, Taylor RM (1985) The influence of surgical operations on components of the human immune system. Br J Surg 72:771–776
Eggermont AM, Steller EP, Sugerbaker PH (1987) Laparotomy enhances intraperitoneal tumor growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery 102:71–78
Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL (1999) Increased tumor establishment and growth after open versus laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc 13:233–235
Belizon A, Balik E, Horst P, Feingold D, Arnell T, Azarani T, Cekic V, Skitt R, Kumara S, Whelan RL (2008) Persistent elevation of plasma vascular endothelial growth factor levels during the first month after minimally invasive colorectal resection. Surg Endosc 22:287–297
Shantha Kumara HMC, Feingold D, Kalady M, Dujovny N, Senagore A, Hyman N, Cekic V, Whelan RL (2009) Colorectal resection is associated with persistent proangiogenic plasma protein changes: postoperative plasma stimulates in vitro endothelial cell growth, migration, and invasion. Ann Surg 249:973–977
Folkman J (1990) What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 82:4–6
Folkman J (1974) Tumor angiogenesis factor. Cancer Res 34:2109–2113
Kissmeyer-Nielsen P, Vinter-Jensen L, Smerup M (1996) Effects of long-term epidermal growth factor treatment on the normal rat colon. Gut 38:582–586
Yokoi K, Thaker PH, Yazici S, Rebhun RR, Nam DH, He J, Kim SJ, Abbruzzese JL, Hamilton SR, Fidler IJ (2005) Dual inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation by AEE788 reduces growth and metastasis of human colon carcinoma in an orthotopic nude mouse model. Cancer Res 65:3716–3725
Yoon SS, Kim SH, Gonen M, Heffernan NM, Detwiller KY, Jarnagin WR, D’Angelica M, Blumgart LH, Tanabe KK, Dematteo RP (2006) Profile of plasma angiogenic factors before and after hepatectomy for colorectal cancer liver metastases. Ann Surg Oncol 13:353–362
Rikimaru K, Tadokoro K, Yamamoto T, Enomoto S, Tsuchida N (1992) Gene amplification and overexpression of epidermal growth factor receptor in squamous cell carcinoma of the head and neck. Head Neck 14:8–13
Reeves JR, Smith G, Keith WN, Ozanne BW, Cooke TG, Stanton PD (1996) Stanton quantitative estimation of epidermal growth factor receptor and c-erbB-2 in human breast cancer. Cancer Res 56:3823–3830
Veale D, Kerr N, Gibson GJ, Kelly PJ, Harris AL (1993) The relationship of quantitative epidermal growth factor receptor expression in non-small cell lung cancer to long term survival. Br J Cancer 68:162–165
Hollstein MC, Smits AM, Galiana C, Yamasaki H, Bos JL, Mandard A, Partensky C, Montesano R (1988) Amplification of epidermal growth factor receptor gene but no evidence of ras mutations in primary human esophageal cancers. Cancer Res 48:5119–5123
Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchier M, Beger HG (1992) Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest 90:1352–1360
Ishikawa J, Maeda S, Umezu K, Sugiyama T, Kamidono S (1990) Amplification and overexpression of the epidermal growth factor receptor gene in human renal-cell carcinoma. Int J Cancer 45:1018–1021
Kim JW, Kim YT, Kim DK, Song CH, Lee JW (1996) Expression of epidermal growth factor receptor in carcinoma of the cervix. Gynecol Oncol 60:283–287
Fischer-Colbrie J, Witt A, Heinzl H, Speiser P, Czerwenka K, Sevelda P, Zeillinger R (1997) EGFR and steroid receptors in ovarian carcinoma: comparison with prognostic parameters and outcome of patients. Anticancer Res 17:613–619
Olapade-Olaopa EO, Moscatello DK, MacKay EH, Horsburgh T, Sandhu DP, Terry TR, Wong AJ, Habib FK (2000) Evidence for the differential expression of a variant EGF receptor protein in human prostate cancer. Br J Cancer 82:186–194
Chow NH, Liu HS, Lee EI, Chang CJ, Chan SH, Cheng HL, Tzai TS, Lin JS (1997) Significance of urinary epidermal growth factor and its receptor expression in human bladder cancer. Anticancer Res 17:1293–1296
Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY (1993) Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell 4:121–133
Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS (1997) Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol 151:1523–1530
Disclosures
Michael J. Grieco, H. M. C. Shantha Kumara, Raymond Baxter, Nadav Dujovny, Matthew F. Kalady, Vesna Cekic, Martin Luchtefeld, and Richard L. Whelan have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grieco, M.J., Shantha Kumara, H.M.C., Baxter, R. et al. Minimally invasive colorectal resection is associated with a rapid and sustained decrease in plasma levels of epidermal growth factor (EGF) in the colon cancer setting. Surg Endosc 24, 2617–2622 (2010). https://doi.org/10.1007/s00464-010-1018-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1018-x