Abstract
Background
Inflammation and wound healing play critical roles in the integration of biologic and biodegradable meshes (BMs) at hernia repair sites. Monocytes/macrophages (M/MØs) are key cells controlling inflammation and wound healing. These cells release inflammatory cytokines and growth factors such as interleukin (IL)-1β, IL-6, IL-8, and vascular endothelial growth factor (VEGF) upon activation. Although BMs have been increasingly used in hernia repairs worldwide, to date, investigations of inflammatory responses to various BMs have been limited.
Methods
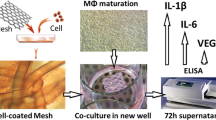
Mesh samples of three acellular human dermis-derived biologic meshes (AlloDerm, AlloMax, FlexHD) and one biodegradable synthetic mesh (Bio-A) were placed in 96-well plates. Human peripheral blood mononuclear cells (PBMCs) were isolated from six healthy subjects, added to each well, and incubated for 7 days. Culture supernatants were assayed for IL-1β, IL-6, IL-8, and VEGF levels using a multiplex bead-base immunoassay system (Bio-Plex).
Results
All four meshes induced cytokine expression from activated M/MØs to varying degrees in vitro. FlexHD induced significantly more IL-1β (2,591 pg/ml) than AlloMax (517 pg/ml), AlloDerm (48 pg/ml), or Bio-A (28 pg/ml) (p < 0.001). AlloMax stimulated a significantly greater quantity of IL-6 (38,343 pg/ml) than FlexHD (19,317 pg/ml), Bio-A (191 pg/ml), or AlloDerm (103 pg/ml) (p < 0.05). Interleukin-8 and VEGF displayed trends similar to that of IL-6. There were no significant differences in cytokine production between AlloDerm and Bio-A.
Conclusion
This study demonstrated that human macrophages are activated by human dermis-derived biologic and biodegradable meshes in vitro. A wide range of cytokine and growth factor induction was seen among the different mesh products. These differences in M/MØ activation may be related to the proprietary processing technologies of the studied meshes. The study results raise the possibility that these differences in M/MØ activation could indicate varying intensities of inflammation that control integration of different biologic meshes at the sites of hernia repair.

Similar content being viewed by others
References
Gray SH, Hawn MT, Itani KM (2008) Surgical progress in inguinal and ventral incisional hernia repair. Surg Clin North Am 88:17–26, vii
Rutkow IM (2003) Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am 83:1045–1051, v–vi
Kingsnorth A, LeBlanc K (2003) Hernias: inguinal and incisional. Lancet 362:1561–1571
Bachman S, Ramshaw B (2008) Prosthetic material in ventral hernia repair: how do I choose? Surg Clin North Am 88:101–112, ix
Udwadia T (2006) Inguinal hernia repair: the total picture. J Min Access Surg 2:144–146
Jin J, Rosen MJ, Blatnik J, McGee MF, Williams CP, Marks J, Ponsky J (2007) Use of acellular dermal matrix for complicated ventral hernia repair: does technique affect outcomes? J Am Coll Surg 205:654–660
Milburn ML, Holton LH, Chung TL, Li EN, Bochicchio GV, Goldberg NH, Silverman RP (2008) Acellular dermal matrix compared with synthetic implant material for repair of ventral hernia in the setting of perioperative Staphylococcus aureus implant contamination: a rabbit model. Surg Infect Larchmt 9:433–442
Saettele TM, Bachman SL, Costello CR, Grant SA, Cleveland DS, Loy TS, Kolder DG, Ramshaw BJ (2007) Use of porcine dermal collagen as a prosthetic mesh in a contaminated field for ventral hernia repair: a case report. Hernia 11:279–285
Hiles M, Record Ritchie RD, Altizer AM (2009) Are biologic grafts effective for hernia repair? A systematic review of the literature. Surg Innov 16:26–37
Schuster R, Singh J, Safadi BY, Wren SM (2006) The use of acellular dermal matrix for contaminated abdominal wall defects: wound status predicts success. Am J Surg 192:594–597
Shaikh FM, Giri SK, Durrani S, Waldron D, Grace PA (2007) Experience with porcine acellular dermal collagen implant in one-stage tension-free reconstruction of acute and chronic abdominal wall defects. World J Surg 31:1966–1972; discussion 1973–1964, 1975
Bellows CF, Albo D, Berger DH, Awad SS (2007) Abdominal wall repair using human acellular dermis. Am J Surg 194:192–198
Badylak SF (2004) Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol 12:367–377
Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM (2008) Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14:1835–1842
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964
Schachtrupp A, Klinge U, Junge K, Rosch R, Bhardwaj RS, Schumpelick V (2003) Individual inflammatory response of human blood monocytes to mesh biomaterials. Br J Surg 90:114–120
Schutte RJ, Parisi-Amon A, Reichert WM (2009) Cytokine profiling using monocytes/macrophages cultured on common biomaterials with a range of surface chemistries. J Biomed Mater Res A 88:128–139
Orenstein S, Kaur M, Klueh U, Kreutzer D, Novitsky Y (2009) Activation of human monocytes by human biologic meshes in vitro (abstract). J Surg Res 151:290
Butler CE (2006) The role of bioprosthetics in abdominal wall reconstruction. Clin Plast Surg 33:199–211, v–vi
Jansen PL, Mertens Pr P, Klinge U, Schumpelick V (2004) The biology of hernia formation. Surgery 136:1–4
Sandor M, Xu H, Connor J, Lombardi J, Harper JR, Silverman RP, McQuillan DJ (2008) Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng Part A 14:2021–2031
Xu H, Wan H, Sandor M, Qi S, Ervin F, Harper JR, Silverman RP, McQuillan DJ (2008) Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng Part A 14:2009–2019
Kaback LA, Smith TJ (1999) Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1 beta in human orbital fibroblasts: potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 84:4079–4084
Oncul O, Yildiz S, Gurer US, Yeniiz E, Qyrdedi T, Top C, Gocer P, Akarsu B, Cevikbas A, Cavuslu S (2007) Effect of the function of polymorphonuclear leukocytes and interleukin-1 beta on wound healing in patients with diabetic foot infections. J Infect 54:250–256
Yamada Y, Itano N, Hata K, Ueda M, Kimata K (2004) Differential regulation by IL-1 beta and EGF of expression of three different hyaluronan synthases in oral mucosal epithelial cells and fibroblasts and dermal fibroblasts: quantitative analysis using real-time RT-PCR. J Invest Dermatol 122:631–639
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601
Barton BE (1997) IL-6: insights into novel biological activities. Clin Immunol Immunopathol 85:16–20
Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F (2003) Principles of interleukin (IL)-6-type cytokine signaling and its regulation. Biochem J 374:1–20
Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C (1994) Macrophages and angiogenesis. J Leukoc Biol 55:410–422
Novitsky YW, Litwin DE, Callery MP (2004) The net immunologic advantage of laparoscopic surgery. Surg Endosc 18:1411–1419
Labler L, Rancan M, Mica L, Harter L, Mihic-Probst D, Keel M (2009) Vacuum-assisted closure therapy increases local interleukin-8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma 66:749–757
Mukaida N, Harada A, Matsushima K (1998) Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev 9:9–23
Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR (1992) Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 3:211–220
Yoo SA, Bae DG, Ryoo JW, Kim HR, Park GS, Cho CS, Chae CB, Kim WU (2005) Arginine-rich antivascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-alpha and IL-6 by human monocytes. J Immunol 174:5846–5855
Lee TH, Avraham H, Lee SH, Avraham S (2002) Vascular endothelial growth factor modulates neutrophil transendothelial migration via upregulation of interleukin-8 in human brain microvascular endothelial cells. J Biol Chem 277:10445–10451
Badylak SF, Gilbert TW (2008) Immune response to biologic scaffold materials. Semin Immunol 20:109–116
Gilbert TW, Sellaro TL, Badylak SF (2006) Decellularization of tissues and organs. Biomaterials 27:3675–3683
Blackwood KA, McKean R, Canton I, Freeman CO, Franklin KL, Cole D, Brook I, Farthing P, Rimmer S, Haycock JW, Ryan AJ, MacNeil S (2008) Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials 29:3091–3104
Acknowledgment
This study was funded in part by institutional support from the University of Connecticut Health Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orenstein, S.B., Qiao, Y., Kaur, M. et al. Human monocyte activation by biologic and biodegradable meshes in vitro. Surg Endosc 24, 805–811 (2010). https://doi.org/10.1007/s00464-009-0664-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-009-0664-3