Abstract
Introduction
Plasma vascular endothelial growth factor (VEGF) levels are increased after surgery and may stimulate tumor growth after cancer resection. Angiopoietin 1 (Ang 1) and Ang 2 are proteins that impact VEGF-related angiogenesis (VRA). Ang 1 stabilizes mature vessels and inhibits VRA, whereas Ang 2 destabilizes vessels and promotes VRA. The ratio of Ang 1 to Ang 2 reflects the net effect; a low ratio promotes VRA. This study’s purpose was to determine the impact of open and minimally invasive (MIS) colorectal resection (CR) for benign indications on plasma Ang 1 and 2 levels.
Methods
A total of 30 patients operated by MIS and 26 operated by open procedure were studied. Plasma was obtained preoperatively (PO) and on postoperative days (POD) 1 and 3. Plasma Ang 1 and Ang 2 levels were assessed via enzyme-linked immunosorbent assay (ELISA) in duplicate. Data were compared using Wilcoxon’s matched-pair test and the Mann–Whitney U-test (significance p < 0.05).
Results
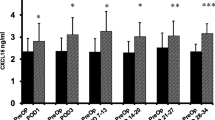
Indications, types of resection, and morbidity for the groups were similar. The mean MIS incision length was 4.7 ± 1.6 cm while it was 16.8 ± 7.1 cm for the open group (p = 0.0001). For both groups Ang 2 levels were significantly higher and the Ang 1 to Ang 2 ratio was significantly lower on POD 1 and 3 compared with preoperative results. Ang 1 levels were significantly decreased on POD 1 and 3 in the MIS group but only on POD 1 in the open group. For unclear reasons, preoperative Ang 1 levels and Ang 1 to Ang 2 ratios were significantly different between the groups, which precludes comparison of the postoperative results between groups.
Conclusion
CR for benign pathology results in higher Ang 2 levels, lower Ang 1 levels, and lower Ang 1 to Ang 2 ratios early after surgery. These alterations are proangiogenic. These results, plus the already noted VEGF increases, suggest that surgery results in proangiogenic plasma protein changes that may stimulate tumor growth early after surgery. The duration of the Ang 1 and 2 changes needs to be determined.



Similar content being viewed by others
References
Bessey PQ (1994) Metabolic response to critical illness. In: Meakins JL (ed) Surgical infections diagnosis and treatment. Scientific American, New York pp 36–42
Michie HR (1996) Metabolism of sepsis and multiple organ failure. World J Surg 20:460–464
Kirman I, Cekic V, Poltaratskaia N, Sylla P, Jain S, Forde KA, Whelan RL (2005) Open surgery induces a dramatic decrease in circulating intact IGFBP-3 in patients with colorectal cancer not seen with laparoscopic surgery. Surg Endosc 19:55–59
Schwenk W, Jacobi C, Mansmann U, Böhm B, Müller JM (2000) Inflammatory response after laparoscopic and conventional colorectal resections. Langenbeck Arch Surg 385:2–9
Kirman I, Jain S, Cekic V, Belizon A, Balik E, Sylla P, Arnell T, Forde KA, Whelan RL (2006) Altered plasma matrix metalloproteinase-9/tissue metalloproteinase-1 concentration during the early post-operative period in patients with colorectal cancer. Surg Endosc 20:482–486
Belizon A, Horst P, Balik E, Feingold D, Arnell T, Azarani T, Cekic V, Skitt R, Kumara S, Whelan RL (2008) Persistent elevation of plasma VEGF levels during the first month following minimally invasive colorectal resection. Surg Endosc 22:287–297
Grad S, Ertel W, Keel M, Infanger M, Vonderschmitt DJ, Maly FE (1998) Strongly enhanced serum levels of vascular endothelial growth factor (VEGF) after polytrauma and burn. Clin Chem Lab Med 36(6):379–383
Wu FP, Hoekman K, Sietses C, von Blomberg BM, Meijer S, Bonjer HJ, Cuesta MA (2004) Systemic and peritoneal angiogenic response after laparoscopic or conventional colon resection in cancer patients: a prospective, randomized trial. Dis Colon Rectum 47(10):1670–1674
Karayiannakis AJ, Zbar A, Polychronidis A, Simopoulos C (2003) Serum and drainage fluid vascular endothelial growth factor levels in early surgical wounds. Eur Surg Res 35(6):492–496
Folkman J (1997) Addressing tumor blood vessels. Nat Biotechnol 15:510
Kampfer H, Pfeilschifter J, Frank S (2001) Expressional regulation of angiopoetin-1 and -2 and the Tie-1 and -2 receptor tyrosine kinases during cutaneous wound healigng: a comparative study of normal and impaired repair. Lab Invest 81(3):361–373
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277(5322):55–60
Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD (1998) Increased vascularization in mice overexpressing angiopoietin-1. Science 282(5388):468–471
Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC (1999) Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest 79(2):213–223
Teichert-Kuliszewska K, Maisonpierre PC, Jones N, Campbell AI, Master Z, Bendeck MP, Alitalo K, Dumont DJ, Yancopoulos GD, Stewart DJ (2001) Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res 49(3):659–670
Tait CR, Jones PF (2004) Angiopoetins in tumors: the angiogenic switch. J Pathol 204:1–10
Liekens S, DeClercq E, Neyts J (2001) Angiogenesis: regulators and clinical applications (commentary). Biochem Pharmacol 61:253–270
Hormbrey E, Han C, Roberts A, McGrouther DA, Harris AL (2003) The relationship of human wound vascular endothelial growth factor (VEGF) after breast cancer surgery to circulating VEGF and angiogenesis. Clin Cancer Res 9(12):4332–4339
Gudehithlu KP, Ahmed N, Wu H, Litbarg NO, Garber SL, Arruda JA, Dunea G, Singh AK (2005) Antagonism of vascular endothelial growth factor results in microvessel attrition and disorganization of wound tissue. J Lab Clin Med 145(4):194–203
Belizon A, Balik E, Feingold DL, Bessler M, Arnell TD, Forde KA, Horst PK, Jain S, Cekic V, Kirman I, Whelan RL (2006) Major abdominal surgery increases plasma levels of vascular endothelial growth factor; open more so than minimally invasive methods. Ann Surg 244(5):792–798
Kumara S, Kirman I, Feingold D, Cekic V, Nasar A, Arnell T, Balik E, Hoffman A, Baxter R, Conte S, Whelan RL (2008) Perioperative GMCSF limits the proangiogenic plasma protein changes associated with colorectal cancer resection. Eur J Surg Oncol [Epub a head of print]
Kirman I, Cekic V, Poltaratskaia N, Asi Z, Bessler M, Huang EH, Forde KA, Whelan RL (2002) Plasma from patients undergoing major open surgery stimulates in vitro tumor growth: lower insulin-like growth factor binding protein 3 levels may, in part, account for this change. Surgery 132:186–192
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00464-008-0212-6
Rights and permissions
About this article
Cite this article
Shantha Kumara, H.M.C., Hoffman, A., Kim, I.Y. et al. Colorectal resection, both open and laparoscopic-assisted, in patients with benign indications is associated with proangiogenic changes in plasma angiopoietin 1 and 2 levels. Surg Endosc 23, 409–415 (2009). https://doi.org/10.1007/s00464-008-0132-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-008-0132-5