Abstract
Background
Gallstones are a major cause of morbidity, and cholecystectomy is a commonly performed procedure. Minimal invasive procedures, laparoscopic cholecystectomy (LC) and small-incision cholecystectomy (SIC), have replaced the classical open cholecystectomy. No differences have been found in primary outcome measures between LC and SIC, therefore secondary outcome measures have to be considered to determine preferences. The aim of our study was to examine health status applying evidence-based guidelines in LC and SIC in a randomised trial.
Methods
Patients with symptomatic cholecystolithiasis were included in a blind randomised trial. Operative procedures, anaesthesia, analgesics and postoperative care were standardised in order to limit bias. Questionnaires were filled in preoperatively, the first day postoperatively, and at outpatients follow-up at 2, 6 and 12 weeks. In accordance with evidence-based guidelines, the generic short form (SF-36) and the disease-specific gastrointestinal quality-of-life index (GIQLI) questionnaires were used in addition to the body image questionnaire (BIQ).
Results
A total of 257 patients were randomised between LC (120) and SIC (137). Analyses were performed according to intention-to-treat (converted procedures included) and also distinguishing converted from minimal invasive (nonconverted) procedures. Questionnaires were obtained with a response rate varying from 87.5% preoperatively to 77.4% three months postoperatively. Except for two time-specific measurements in one SF-36 subscale, there were no differences between LC and SIC. There were significant differences in several subscales in all three questionnaires comparing minimal invasive versus converted procedures.
Conclusions
Applying adequate methodological quality and evidence-based guidelines (by using SF-36 and GIQLI), there are no significant differences in health status between LC and SIC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cholecystectomy is a commonly performed procedure in patients with symptomatic cholecystolithiasis. With an estimated incidence up to 2.17 per thousand inhabitants [1, 2], and 500,000 cholecystectomies performed annually in the USA [3] and 21,000 in The Netherlands (an incidence of 1.31 per thousand inhabitants) [4, 5], gallstones are a major cause of morbidity in the Western world. During the 1980s, the preferred surgical technique for cholecystectomy changed from the classical open procedure to a smaller incision approach [6, 7] and eventually to laparoscopic cholecystectomy. Although evidence of superiority was never delivered, the laparoscopic technique was accepted as the gold-standard procedure by consensus [3].
Multiple randomised trials comparing laparoscopic (LC) and small-incision cholecystectomy (SIC) have been performed and results are inconsistent. Some favour the SIC technique, others favour the LC technique, and many take a neutral position. All these randomised trials are included in our Cochrane review. Our review showed no differences in primary outcome measures between LC and SIC [8].
In comparing (surgical) treatments, primary outcome measures (mortality and severe complications) have to be considered prior to secondary outcome measures. As no significant differences between LC and SIC in primary outcome measures were found [8], it is justified to consider health status, an important secondary outcome measure. Frequently, quality of life is confused with health status. Quality of life measures the subjective judgment of patients about their condition, while health status refers to the impact of disease on patients’ lives in the physical, psychological and social domains.
Questionnaires, both generic and condition-specific, have been shown to be useful in measuring changes in health status after cholecystectomy [9–11]. Several studies showed that health status was improved, both after LC and open cholecystectomy in patients suffering socially disabling uncomplicated symptomatic cholecystolithiasis [12–14]. Differences between the open and laparoscopic technique are not clear [15], although some studies found superior results using the laparoscopic technique [16, 17].
To date, differences in health status between LC and SIC are not very well examined [18–20]. Moreover, as the previous studies did not use the appropriate questionnaires as advised by evidence-based guidelines, there had been no possibility to correctly find differences in health status between both operating techniques.
The gastrointestinal quality of life index (GIQLI) and the short form (SF-36) are frequently used and validated questionnaires (disease-specific and generic, respectively) and are most suitable for evaluating patients’ functional recovery after cholecystectomy [21].
Objective
The aim of our study was to examine differences in health status in patients with symptomatic cholecystolithiasis before and after LC and SIC in a blinded randomised clinical trial. We used the GIQLI and the SF-36 questionnaires, as recommended by evidence-based guidelines [21].
Methods
All patients with symptomatic cholecystolithiasis visiting the outpatients clinic of the St. Elisabeth hospital in Tilburg were considered for inclusion in a blind randomised trial comparing laparoscopic and small-incision cholecystectomy. Verbal and written informed consent was obtained from each patient, and patients were consecutively listed for elective cholecystectomy. Health status was a secondary outcome measure as part of the randomised clinical trial.
Sample size
No differences in primary outcome measures (mortality and complications) were expected between LC and SIC [8]. Consequently, a secondary outcome measure should be used to decide on preferences between both techniques. We decided to focus on costs between both techniques as the most important secondary outcome measure. Based on an anticipated difference of 10% in direct costs 120 patients had to be included in each group. However, multiple outcome measures including health status were evaluated in this randomised trial.
Based on a previous study [18], it was calculated that 128 patients were needed in each group to detect a difference of 5 points (assuming a standard deviation of 20) in the gastrointestinal quality-of-life index (GIQLI) questionnaire with a type I error of 0.05 and a power of 0.8.
Randomisation
As randomised trials with high bias risk may overestimate intervention effects [22], results of randomised trials with low bias risk are considered more reliable. Therefore, attention is warranted for correct generation of the allocation sequence, allocation concealment, blinding, and follow-up.
A random-number table was used for the generation of the allocation sequence and allocation concealment was guaranteed by using sealed envelopes. To eliminate bias caused by preoperative expectations, patients were randomised in the operation theatre after induction of anaesthesia. A telephone call to the secretary office was made and an employee opened an envelope. All patient data were recorded in a case record form, with the procedure reported as ‘trial cholecystectomy’. Wounds and port sites were dressed with identical opaque dressings, stained using iodine, regardless of the surgical procedure performed, to allow blinding for patient, nurses, and physicians during the postoperative period. The type of operation was revealed just before discharge.
No patients were lost to follow-up. Operative procedures were standardised apart from using a laparoscopic or small-incision technique. Anaesthesia, postoperative care and analgesic use were also standardised.
Inclusion and exclusion criteria
Inclusion criteria were: male or female patients with symptomatic cholecystolithiasis, age 18 years or older at recruitment, with reasonable to good health according to American Society of Anaesthesiologists (ASA) classification (ASA I or II) [23], no known relevant allergies and a signed letter of informed consent.
Exclusion criteria were: age younger than 18 years, choledocholithiasis (icterus, acholic faeces and/or bilirubine twice normal range), cholangitis, known pregnancy, moderate to severe systemic disease (ASA III and higher), known cirrhosis of the liver, history of abdominal malignancy, previous upper abdominal surgery (precluding laparoscopic approach), psychiatric disease, or another reason (e.g. lack of knowledge of the Dutch language) for making follow-up or completion of questionnaires unreliable.
Obesity was indexed but not considered an exclusion criterion [24]. Recovery after successful endoscopic treatment of choledocholithiasis was not a contraindication. Acute cholecystitis is a different disease with other complication rates, morbidity, and conversion rates, and patients suffering acute cholecystitis were, therefore, not included.
Surgical procedures
The policy in our hospital was not to perform operative cholangiography in any patient in elective cholecystectomy. All patients had nasogastric intubations during the operation that were removed immediately afterwards. Bladder drainage was not performed. Abdominal wall and skin closure were standardised. In case of technical difficulties or for safety reasons, both laparoscopic and small-incision cholecystectomies were converted to open cholecystectomy by a subcostal incision (>8 cm). Reasons for conversion were registered. The wounds were covered with standard wound dressings as described by Majeed [24] to blind patient and ward personnel postoperatively. We did not use any local anaesthetic technique into the wounds nor intercostal nerve blocks.
Laparoscopic cholecystectomy
Open introduction of trocars was performed in all patients, regardless of previous abdominal surgery. Pneumoperitoneum was created using the subumbilical trocar with an intra-abdominal pressure up to 12 mmHg. Three trocars for instruments were inserted. The dissection of the cystic artery and cystic duct, identifying Calot’s triangle, was performed using a three-point ‘flag’ technique [25]. The cystic duct and artery were clipped and transsected. After complete dissection of the gallbladder, it was removed either through the subumbilical or the subxyphoidal trocar. Fascia defects as a result of the insertion of 10mm trocar and the open introduction of the subumbilical trocar were closed with UR6 vicryl 1.0/2.0® sutures. All instruments, except for the subumbilical trocar, were reusable. No suction drains were left in the subhepatic space at the end of the procedure.
Small-incision cholecystectomy
In the literature most authors used 8 cm (or less) as a cut-off point to differentiate between small-incision and open cholecystectomy [24, 26–32]. Therefore, we performed small-incision cholecystectomy principally through an incision of 6 cm, maximally extended to 8 cm. As part of a separate research question, all patients had a preoperative ultrasound scan and the location of the fundus of the gallbladder was marked on the skin. We used the craniocaudal position of the mark for incision. The mediolateral position of the mark was not used, because in the pilot phase we found that the incision would be too lateral for adequate view of the hilus. The incision was placed over the musculus rectus abdominis. Only standard instruments were used and no special equipment. Access to the peritoneum was obtained by a muscle splitting (and not transsection) technique of the musculus rectus abdominis (like in an open appendectomy). The gallbladder was dissected by a fundus-first technique. If necessary the gallbladder was punctured to remove its liquid contents. The cystic duct and artery were ligated and the gallbladder was removed. No suction drains were left in the subhepatic space at the end of the procedure. Posterior and anterior fascias were closed separately with PDS 3.0® running suture. After wound closure, the length of the incision was measured. When the length exceeded 8 cm, the operation was considered to be a conversion to open cholecystectomy.
Postoperative protocol
Early oral intake and mobilization were encouraged. Patients left the hospital as soon as they felt capable. As patients were admitted at the day of operation, hospital stay was defined as the number of nights (postoperative) in hospital. Shortly before discharge, wound dressings were removed for wound inspection. For logistic reasons, we were not able to blind the surgeon at the patients’ follow-up. Follow-up took place according to a standardised scheme after 2 weeks, 6 weeks, and 3 months. Patients were encouraged to resume work and normal daily activity as soon as they felt capable to do so.
Measurements
In accordance with evidence-based guidelines [21], we decided to use the generic short form (SF-36) and the disease-specific gastrointestinal quality-of-life index (GIQLI) questionnaires. These questionnaires were completed preoperatively, on the first day postoperative and at each follow-up visit after 2 and 6 weeks and after 3 months. In addition, the body image questionnaire (BIQ) was completed preoperatively and at 6 weeks postoperatively in order to estimate differences in the patients’ perception of their body image and cosmetics [33].
The SF-36 is a generic health status questionnaire that has 36 questions to assess eight domains (physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health) [34]. Internal consistency measured by Cronbach’s alpha was shown to be high (above 0.80 in all subscales) [34]. The Dutch version has been validated [35].
The GIQLI is a disease-specific health status measure. It includes both specific questions on gastrointestinal symptoms, for both the upper and the lower gastrointestinal tract, as well as questions on physical, emotional and social capabilities [36]. It is a mixed questionnaire that includes both generic and specific questions. Based on face validity, five subscales are distinguished in addition to a total score. Internal consistency measured by Cronbach’s alpha was shown to be high (above 0.90 in all subscales) [36]. The Dutch version has been validated [37].
The body image questionnaire (BIQ) consists of nine questions evaluating three subscales: body image, cosmetic, and self-confidence. The BIQ has shown to consist of two factors, a body image and a cosmetic factor [33]. The body image scale measures patients’ perception of and satisfaction with their own body and explores patients’ attitudes toward their bodily appearance. The cosmetic scale assesses the degree of satisfaction of patients with respect to the physical appearance of the scar. Additionally, a question is added to assess patients’ self-confidence before and after surgery. Internal validity (measured by Crohnbach’s alpha) reliability coefficients were shown to be high for both the body image (0.80) and cosmetic scales (0.83) [33].
Statistical analysis
Analyses were performed according to the type of operative procedure used, based on the intention to treat principle. Apart from this main analysis, one subgroup analysis was performed: converted procedures (LC and SIC) were compared with minimal invasive procedures (LC and SIC). This subgroup analysis was performed in order to illustrate the sensitivity of the questionnaires. Calculations were made using SPSS version 11.0®.
Repeated measures analysis of variance (ANOVA) was used to evaluate health status differences over time between the two operative techniques.
Additional independent t-tests were performed to test for time-specific differences in scores at the preoperative measurements between two groups in order to check for a correct randomisation procedure. If appropriate, additional independent t-tests were performed to test for other time-specific differences in measurements.
Results
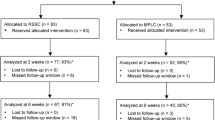
All trial patients were included and operated between January 2001 and March 2004. Leaving unwilling and excluded patients out of consideration, 366 patients initially fulfilled the inclusion criteria and were initially included in the trial. A total of 102 patients were not randomised for a variety of reasons (Fig. 1). After randomizing 264 patients, another seven patients were excluded (after their cholecystectomy) for the following reasons: unwillingness for further participation in the trial (2), intraoperative suspicion of malignancy (2), transfer to another ward not participating in the trial (1), participation in two trials (not in line with the Helsinki declaration) (1), and insufficient knowledge of the Dutch language (1). Excluding the data of these seven patients from our analyses did not affect the results of our questionnaires in any way. A total of 257 patients were left for analysis (LC:120 and SIC:137).
Baseline characteristics and operative results
The groups (LC and SIC) did not differ regarding age, sex, body mass index (BMI) and ASA classification (Table 1). The classical diagnostic symptoms of cholecystolithiasis as well as the duration of these symptoms were also equally distributed in both groups. In addition, the number of patients presenting with complicated gallstone disease who had received treatment by endoscopic retrograde cholangiopancreaticography (ERCP) (and papillotomy) were equally distributed and operated on in a later stage (Table 2).
There was no mortality. There were five intraoperative complications in the LC group compared with three in the SIC group. There were 16 postoperative complications in the LC group and 13 in the SIC group. There were 21 and 16 total complications (intra- and postoperative) in the LC and SIC group, respectively. Of these, 11 and 7 complications were serious in the LC and the SIC group, respectively (Table 3). We did not find a difference in the number or severity of the complications.
Operative time was shorter for SIC compared to LC (60 versus 72 min, respectively; U = 6013.0, p < 0.001). Conversion rates were similar (p = 0.312), with similar reasons for conversion. The follow-up rate between the groups was not statistically different. Follow-up was 91.4–96.3% at six weeks, 82.2–82.8% at three months and 100% at either six weeks or three months. Complaints at follow-up were comparable.
There were no differences in the preoperative measurements of the SF-36 subscales, all the GIQLI subscales, the total GIQLI score and the BIQ subscales.
Health status
The questionnaires were obtained with a response rate varying from 87.5% preoperatively to 77.4% three months postoperatively. The nonresponders did not differ from those who remained in the study with regard to complications (16%), operative time (65 minutes), hospital stay (1.5 days), return to work (3.2 weeks) or baseline scores.
When comparing LC with SIC (intention-to-treat), we found no differences in all SF-36 subscales, except for ‘perceived health change’. There were significant differences favouring the laparoscopic technique (F = 16.054, df = 1; p < 0.001) (Table 4). Performing time-specific analyses, differences were identified at two weeks (p = 0.029) and six weeks (p < 0.001) postoperatively. There were no differences between LC and SIC with regard to the four GIQLI subscales, the total GIQLI score, and the body image subscales.
Subgroup analysis
In checking for differences in preoperative data in the minimal invasive procedures versus conversions comparison, we only found a significant difference in the self-confidence subscale of the body image questionnaire (t = 2.821, df = 207, p = 0.005) with higher self-confidence scores in the minimal invasive operated group (7.08 versus 6.31). No other differences were found in preoperative data.
In order to assess differences between minimal invasive procedures (both laparoscopic and small-incision) and procedures converted to the classical open cholecystectomy, we examined patients’ scores across the follow-up period (Table 5).
There were significant differences in the SF-36 subscales ‘physical functioning’ (F = 4.057, df = 1; p = 0.046) and ‘pain’ (F = 4.391, df = 1; p = 0.038). In the GIQLI questionnaire, there were significant differences in the total score (F = 5.593, df = 1; p = 0.020), and in the ‘physical’ (p = 0.007), ‘social’ (p = 0.003), and ‘mental’ (p = 0.004) subscales. Also, in the BIQ there were significant differences in the ‘body image’ and ‘cosmetic’ subscales between both operative groups, favouring the minimal invasive procedures (F = 13.939, df = 1; p < 0.001). No other differences were found.
Discussion
We have used both generic and disease-specific health status questionnaires and a body image questionnaire to evaluate the effect of LC versus SIC in patients having cholecystectomy for symptomatic cholecystolithiasis. No differences were found between laparoscopic and small-incision cholecystectomies (applying intention-to-treat). However, with regard to minimal invasive or converted procedures, we found significant differences in the ‘physical’ subscales in both SF-36 and GIQLI as well as differences in body image in favour of minimal invasive procedures. The fact that significant differences were found in the ‘physical’ subscales in both questionnaires illustrates construct validity between both health status instruments.
Literature
A few other studies have compared health status after LC and SIC [18–20]. Two studies found that the laparoscopic technique was associated with a more rapid improvement in health status after cholecystectomy compared with the small-incision technique [18, 19]. One study found no differences at all between both techniques [20]. However, it is difficult to draw conclusions from three studies that used different questionnaires and suffer several methodological flaws. None of the mentioned studies combined the SF-36 and GIQLI as advised by evidence-based guidelines [21].
Barkun studied 35 and 23 patients in the LC and SIC groups, respectively, and used the same GIQLI as we did in addition to the Nottingham health profile (NHP) and a visual analogue scale (VAS) for health [18]. Allocation concealment was unclear, no blinding was used, and eight dropouts occurred in their rather small, preliminary stopped trial. They used cumulative totals of both GIQLI and NHP data instead of using subscales. Changes in one dimension might be offset by changes in other dimensions. Both questionnaires have more than one dimension (the cumulative total); subscales indeed provide the advantage of additional information on several dimensions. As a rather small number of patients were included (the trial was stopped preliminary), no subscales were assessed, and no considerations were given to the construct or divergent validity of both questionnaires, their conclusion that LC was associated with a significantly quicker return to ‘good health’ seems inappropriate based on their results.
McMahon compared health status in 151 and 148 laparoscopic and small-incision cholecystectomy patients respectively using the SF-36 health survey questionnaire and the hospital anxiety and depression scale (HADS) [19]. Generation of the allocation sequence in their trial was unclear and no blinding was used. They found that patients recovering from LC enjoyed significantly better health 1 and 4 weeks after the operation compared with those recovering from SIC, but no significant difference was found at 12 weeks. The absence of preoperatively baseline measurements and the absence of considerations on the construct or divergent validity of the questionnaires make conclusions about postoperative data uncertain. Differences in SF-36 and HADS correlated with differences in return to domestic and leisure activities, but were not translated in differences in paid activity.
Squirrell used the NHP in 100 patients (50 in each group) preoperatively, and 3 weeks and 6 months postoperatively [20]. This was the only study that used blinding in their methods. Generation of the allocation sequence in their trial was unclear. At no time there was a significant difference between the two groups. The study used a rather small sample size, and unfortunately they did not use a disease-specific questionnaire, but only one generic questionnaire. They concluded that it is necessary to take a broader view of health and not concentrate simply on pain when assessing postoperative recovery.
In our study, no significant differences were found between LC and SIC using both generic and disease-specific health status as well as body image with response in approximately 80% of patients. The response rate of 77.4% at 3 months follow-up may represent a possible source of bias. However, the nonresponders were comparable to those who remained in the study with regard to complications, operative time, hospital stay, return to work, and baseline scores of questionnaires. Moreover, our response rate is in line with the response rates in the studies of Barkun et al. (58%) and McMahon et al. (78%).
We conclude that there are no differences between both operative techniques regarding health status. The only exception is that in the SF-36 subscale perceived health change we found a difference between LC and SIC, which appeared to be caused by the scores at 2 and 6 weeks postoperatively and disappeared at 3 months follow-up. LC patients reported a larger health change. However, in the evaluation of 17 aspects of health status, only one difference was found. Moreover, this difference in perceived health change was not reflected in an earlier return to work in LC. In contrast, SIC patients returned to work quicker than LC patients, although this different was not significant. Therefore, our overall interpretation is that there are no differences between LC and SIC.
The comparable ‘physical’ subscales in SF-36 and GIQLI, which are supposed to measure the same effect, are both significantly different in the minimal invasive versus conversions comparison illustrating construct validity of both questionnaires. Subscales on different subjects in the questionnaires illustrate divergent validity. Significant differences between minimal invasive and converted procedures illustrate that the questionnaires used are able to measure what they are intended to do.
Conclusion
In our randomised trial with adequate generation of the allocation sequence, concealment of allocation, blinding, and follow-up we used both a generic and a disease-specific questionnaire in addition to a body image questionnaire. There is no significant difference in health status measured with SF-36, GIQLI, and BIQ between laparoscopic and small-incision cholecystectomy (applying the intention-to-treat principle). Additional calculations showed a significant difference between minimal invasive LC or SIC procedures and procedures converted to the classical open cholecystectomy.
References
Legorreta AP, Silber JH, Costantino GN, Kobylinski RW, Zatz SL (1993) Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. JAMA 270:1429–1432
Steiner CA, Bass EB, Talamini MA, Pitt HA, Steinberg EP (1994) Surgical rates and operative mortality for open and laparoscopic cholecystectomy in Maryland. N Engl J Med 330:403–408
NIH Consensus conference (1993) Gallstones and laparoscopic cholecystectomy. JAMA 269:1018–1024
Olsen DO (1991) Laparoscopic cholecystectomy. Am J Surg 161:339–344
Roslyn JJ, Binns GS, Hughes EF, Saunders-Kirkwood K, Zinner MJ, Cates JA (1993) Open cholecystectomy. A contemporary analysis of 42,474 patients. Ann Surg 218:129–137
Dubois F, Berthelot B (1982) [Cholecystectomy through minimal incision]. Nouv Presse Med 11:1139–1141
Goco IR, Chambers LG (1983) “Mini-cholecystectomy” and operative cholangiography. A means of cost containment. Am Surg 49:143–145
Keus F, de Jong JAF, Gooszen HG, van Laarhoven CJHM (2006) Laparoscopic versus small-incision cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 18(4):CD006229
Jones KR, Burney RE, Peterson M, Christy B (1998) Measuring health-status improvement after surgery: experience with the SF-36. Semin Nurse Manag 6:139–143
Cleary PD, Greenfield S, McNeil BJ (1991) Assessing quality of life after surgery. Control Clin Trials 12:189S–203S
Bardsley MJ, Venables CW, Watson J, Goodfellow J, Wright PD (1992) Evidence for validity of a health status measure in assessing short term outcomes of cholecystectomy. Qual Health Care 1:10–14
Eriksen JR, Kristiansen VB, Hjortso NC, Rosenberg J, Bisgaard T (2005) [Effect of laparoscopic cholecystectomy on the quality of life of patients with uncomplicated socially disabling gallstone disease]. Ugeskr Laeger 167:2654–2656
Quintana JM, Arostegui I, Cabriada J, Lopez dT I, Perdigo L (2003) Predictors of improvement in health-related quality of life in patients undergoing cholecystectomy. Br J Surg 90:1549–1555
Berger MY, Olde Hartman TC, van der Velden JJ, Bohnen AM (2004) Is biliary pain exclusively related to gallbladder stones? A controlled prospective study. Br J Gen Pract 54:574–579
Quintana JM, Cabriada J, Arostegui I, Lopez dT I, Bilbao A (2003) Quality-of-life outcomes with laparoscopic vs open cholecystectomy. Surg Endosc 17:1129–1134
Topcu O, Karakayali F, Kuzu MA, Ozdemir S, Erverdi N, Elhan A, Aras N (2003) Comparison of long-term quality of life after laparoscopic and open cholecystectomy. Surg Endosc 17:291–295
Velanovich V (2000) Laparoscopic vs open surgery: a preliminary comparison of quality-of-life outcomes. Surg Endosc 14:16–21
Barkun JS, Barkun AN, Sampalis JS, Fried G, Taylor B, Wexler MJ, Goresky CA, Meakins JL (1992) Randomised controlled trial of laparoscopic versus mini cholecystectomy. The McGill Gallstone Treatment Group. Lancet 340:1116–1119
McMahon AJ, Russell IT, Baxter JN, Ross S, Anderson JR, Morran CG, Sunderland G, Galloway D, Ramsay G, O’Dwyer PJ (1994) Laparoscopic versus minilaparotomy cholecystectomy: a randomised trial. Lancet 343:135–138
Squirrell DM, Majeed AW, Troy G, Peacock JE, Nicholl JP, Johnson AG (1998) A randomized, prospective, blinded comparison of postoperative pain, metabolic response, and perceived health after laparoscopic and small incision cholecystectomy. Surgery 123:485–495
Korolija D, Sauerland S, Wood-Dauphinee S, Abbou CC, Eypasch E, Caballero MG, Lumsden MA, Millat B, Monson JR, Nilsson G, Pointner R, Schwenk W, Shamiyeh A, Szold A, Targarona E, Ure B, Neugebauer E (2004) Evaluation of quality of life after laparoscopic surgery: evidence-based guidelines of the European Association for Endoscopic Surgery. Surg Endosc 18:879–897
Schulz KF, Chalmers I, Hayes RJ, Altman DG (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273:408–412
Davis JE, Sugioka K (1987) Selecting the patient for major ambulatory surgery. Surgical and anesthesiology evaluations. Surg Clin North Am 67:721–732
Majeed AW, Troy G, Nicholl JP, Smythe A, Reed MW, Stoddard CJ, Peacock J, Johnson AG (1996) Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet 347:989–994
Rocko JM, Di Gioia JM (1981) Calot’s triangle revisited. Surg Gynecol Obstet 153:410–414
Grande M, Tucci GF, Adorisio O, Barini A, Rulli F, Neri A, Franchi F, Farinon AM (2002) Systemic acute-phase response after laparoscopic and open cholecystectomy. Surg Endosc 16:313–316
Coelho JC, de Araujo RP, Marchesini JB, Coelho IC, de Araujo LR (1993) Pulmonary function after cholecystectomy performed through Kocher’s incision, a mini-incision, and laparoscopy. World J Surg 17:544–546
McGinn FP, Miles AJ, Uglow M, Ozmen M, Terzi C, Humby M (1995) Randomized trial of laparoscopic cholecystectomy and mini-cholecystectomy. Br J Surg 82:1374–1377
Ros A, Gustafsson L, Krook H, Nordgren CE, Thorell A, Wallin G, Nilsson E (2001) Laparoscopic cholecystectomy versus mini-laparotomy cholecystectomy: a prospective, randomized, single-blind study. Ann Surg 234:741–749
Secco GB, Cataletti M, Bonfante P, Baldi E, Davini MD, Biasotti B, Ravera G, Ferraris R (2002) [Laparoscopic versus mini-cholecystectomy: analysis of hospital costs and social costs in a prospective randomized study]. Chir Ital 54:685–692
Tate JJ, Lau WY, Leung KL, Li AK (1993) Laparoscopic versus mini-incision cholecystectomy. Lancet 341:1214–1215
Walker CB, Bruce DM, Heys SD, Gough DB, Binnie NR, Eremin O (1999) Minimal modulation of lymphocyte and natural killer cell subsets following minimal access surgery. Am J Surg 177:48–54
Dunker MS, Stiggelbout AM, van Hogezand RA, Ringers J, Griffioen G, Bemelman WA (1998) Cosmesis and body image after laparoscopic-assisted and open ileocolic resection for Crohn’s disease. Surg Endosc 12:1334–1340
Ware JE Jr., Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, Sprangers MA, te Velde A, Verrips E (1998) Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 51:1055–1068
Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmulling C, Neugebauer E, Troidl H (1995) Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg 82:216–222
Nieveen van Dijkum EJM, Terwee CB, Oosterveld P, van der Meulen JHP, Gouma DJ, de Haes JCJM (2000) Validation of the gastrointestinal quality of life index for patients with potentially operable periampullary carcinoma. Br J Surg 87:110–115
Egger M, Juni P, Bartlett C (2001) Value of flow diagrams in reports of randomized controlled trials. JAMA 285:1996–1999
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration ISRCTN Register, number ISRCTN67485658
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Keus, F., de Vries, J., Gooszen, H.G. et al. Laparoscopic versus small-incision cholecystectomy: Health status in a blind randomised trial. Surg Endosc 22, 1649–1659 (2008). https://doi.org/10.1007/s00464-007-9675-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-007-9675-0