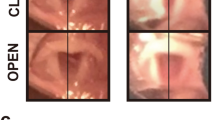
Abstract
Iatrogenic recurrent laryngeal nerve (RLN) injury is a morbid complication of anterior neck surgical procedures. Existing treatments are predominantly symptomatic, ranging from behavioral therapy to a variety of surgical approaches. Though laryngeal reinnervation strategies often provide muscle tone to the paralyzed vocal fold (VF), which may improve outcomes, there is no clinical intervention that reliably restores true physiologic VF movement. Moreover, existing interventions neglect the full cascade of molecular events that affect the entire neuromuscular pathway after RLN injury, including the intrinsic laryngeal muscles, synaptic connections within the central nervous system, and laryngeal nerve anastomoses. Systematic investigations of this pathway are essential to develop better RLN regenerative strategies. Our aim was to develop a translational mouse model for this purpose, which will permit longitudinal investigations of the pathophysiology of iatrogenic RLN injury and potential therapeutic interventions. C57BL/6J mice were divided into four surgical transection groups (unilateral RLN, n = 10; bilateral RLN, n = 2; unilateral SLN, n = 10; bilateral SLN, n = 10) and a sham surgical group (n = 10). Miniaturized transoral laryngoscopy was used to assess VF mobility over time, and swallowing was assessed using serial videofluoroscopy. Histological assays were conducted 3 months post-surgery for anatomical investigation of the larynx and laryngeal nerves. Eight additional mice underwent unilateral RLN crush injury, half of which received intraoperative vagal nerve stimulation (iVNS). These 8 mice underwent weekly transoral laryngoscopy to investigate VF recovery patterns. Unilateral RLN injury resulted in chronic VF immobility but only acute dysphagia. Bilateral RLN injury caused intraoperative asphyxiation and death. VF mobility was unaffected by SLN transection (unilateral or bilateral), and dysphagia (transient) was evident only after bilateral SLN transection. The sham surgery group retained normal VF mobility and swallow function. Mice that underwent RLN crush injury and iVNS treatment demonstrated accelerated and improved VF recovery. We successfully developed a mouse model of iatrogenic RLN injury with impaired VF mobility and swallowing function that can serve as a clinically relevant platform to develop translational neuroregenerative strategies for RLN injury.










Similar content being viewed by others
References
Chandrasekhar SS, Randolph GW, Seidman MD, Rosenfeld RM, Angelos P, Barkmeier-Kraemer J, Benninger MS, Blumin JH, Dennis G, Hanks J, Haymart MR, Kloos RT, Seals B, Schreibstein JM, Thomas MA, Waddington C, Warren B, Robertson PJ, Surgery AAoO-HaN. Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg. 2013;148(6 Suppl):S1–37. https://doi.org/10.1177/0194599813487301.
Mattsson P, Hydman J, Svensson M. Recovery of laryngeal function after intraoperative injury to the recurrent laryngeal nerve. Gland Surg. 2015;4(1):27–35. https://doi.org/10.3978/j.issn.2227-684X.2015.01.10.
Brunner E, Friedrich G, Kiesler K, Chibidziura-Priesching J, Gugatschka M. Subjective breathing impairment in unilateral vocal fold paralysis. Folia Phoniatr Logop. 2011;63(3):142–6. https://doi.org/10.1159/000316320.
Wang B, Yuan J, Chen X, Xu J, Li Y, Dong P. Functional regeneration of the transected recurrent laryngeal nerve using a collagen scaffold loaded with laminin and laminin-binding BDNF and GDNF. Sci Rep. 2016;6:32292. https://doi.org/10.1038/srep32292.
Dionigi G, Wu CW, Kim HY, Rausei S, Boni L, Chiang FY. Severity of recurrent laryngeal nerve injuries in thyroid surgery. World J Surg. 2016;40(6):1373–81. https://doi.org/10.1007/s00268-016-3415-3.
Crumley RL. Laryngeal synkinesis revisited. Ann Otol Rhinol Laryngol. 2000;109(4):365–71. https://doi.org/10.1177/000348940010900405.
Hydman J, Mattsson P. Collateral reinnervation by the superior laryngeal nerve after recurrent laryngeal nerve injury. Muscle Nerve. 2008;38(4):1280–9. https://doi.org/10.1002/mus.21124.
Crumley RL. Mechanisms of synkinesis. Laryngoscope. 1979;89(11):1847–54. https://doi.org/10.1288/00005537-197911000-00020.
Crumley RL. Unilateral recurrent laryngeal nerve paralysis. J Voice. 1994;8(1):79–83.
Kovacic U, Sketelj J, Bajrović FF. Chapter 26: age-related differences in the reinnervation after peripheral nerve injury. Int Rev Neurobiol. 2009;87:465–82. https://doi.org/10.1016/s0074-7742(09)87026-8.
Verdú E, Butí M, Navarro X. The effect of aging on efferent nerve fibers regeneration in mice. Brain Res. 1995;696(1–2):76–82.
Tanaka K, Zhang QL, Webster HD. Myelinated fiber regeneration after sciatic nerve crush: morphometric observations in young adult and aging mice and the effects of macrophage suppression and conditioning lesions. Exp Neurol. 1992;118(1):53–61.
Marawar S, Girardi FP, Sama AA, Ma Y, Gaber-Baylis LK, Besculides MC, Memtsoudis SG. National trends in anterior cervical fusion procedures. Spine. 2010;35(15):1454–9. https://doi.org/10.1097/brs.0b013e3181bef3cb.
Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Kosary C, Yu M, Ruh lJ, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K. SEER Cancer Statistics Review, 1975–2014. 2016. National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2014/. 2017.
Choi JS, Oh SH, An HY, Kim YM, Lee JH, Lim JY. Functional regeneration of recurrent laryngeal nerve injury during thyroid surgery using an asymmetrically porous nerve guide conduit in an animal model. Thyroid. 2014;24(1):52–9. https://doi.org/10.1089/thy.2013.0338.
Kupfer RA, Old MO, Oh SS, Feldman EL, Hogikyan ND. Spontaneous laryngeal reinnervation following chronic recurrent laryngeal nerve injury. Laryngoscope. 2013;123(9):2216–27. https://doi.org/10.1002/lary.24049.
Rosko AJ, Kupfer RA, Oh SS, Haring CT, Feldman EL, Hogikyan ND. Immunohistologic analysis of spontaneous recurrent laryngeal nerve reinnervation in a rat model. Laryngoscope. 2018;128(3):E117–22. https://doi.org/10.1002/lary.27004.
Nishimoto K, Kumai Y, Yumoto E. Paradoxical movement of rat vocal folds following recurrent laryngeal nerve injury. Acta Otolaryngol. 2014;134(11):1164–71. https://doi.org/10.3109/00016489.2014.936625.
Old MO, Oh SS, Feldman E, Hogikyan ND. Novel model to assess laryngeal function, innervation, and reinnervation. Ann Otol Rhinol Laryngol. 2011;120(5):331–8. https://doi.org/10.1177/000348941112000509.
Gould FD, Lammers AR, Ohlemacher J, Ballester A, Fraley L, Gross A, German RZ. The physiologic impact of unilateral recurrent laryngeal nerve (RLN) lesion on infant oropharyngeal and esophageal performance. Dysphagia. 2015;30(6):714–22. https://doi.org/10.1007/s00455-015-9648-8.
Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Fukuhara T, Gierbolini-Norat EM, Thexton AJ, German RZ. Unilateral superior laryngeal nerve lesion in an animal model of dysphagia and its effect on sucking and swallowing. Dysphagia. 2013;28(3):404–12. https://doi.org/10.1007/s00455-013-9448-y.
Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Thexton AJ, German RZ. The effect of unilateral superior laryngeal nerve lesion on swallowing threshold volume. Laryngoscope. 2013;123(8):1942–7. https://doi.org/10.1002/lary.24051.
Ding P, Fung GS, Lin M, Holman SD, German RZ. The effect of bilateral superior laryngeal nerve lesion on swallowing: a novel method to quantitate aspirated volume and pharyngeal threshold in videofluoroscopy. Dysphagia. 2015;30(1):47–56. https://doi.org/10.1007/s00455-014-9572-3.
Lin YC, Dionigi G, Randolph GW, Lu IC, Chang PY, Tsai SY, Kim HY, Lee HY, Tufano RP, Sun H, Liu X, Chiang FY, Wu CW. Electrophysiologic monitoring correlates of recurrent laryngeal nerve heat thermal injury in a porcine model. Laryngoscope. 2015;125(8):E283–90. https://doi.org/10.1002/lary.25362.
Kwak HY, Dionigi G, Kim D, Lee HY, Son GS, Lee JB, Bae JW, Kim HY. Thermal injury of the recurrent laryngeal nerve by THUNDERBEAT during thyroid surgery: findings from continuous intraoperative neuromonitoring in a porcine model. J Surg Res. 2015. https://doi.org/10.1016/j.jss.2015.06.066.
Paniello RC, Rich JT, Debnath NL. Laryngeal adductor function in experimental models of recurrent laryngeal nerve injury. Laryngoscope. 2015;125(2):E67–72. https://doi.org/10.1002/lary.24947.
Woodson GE. Spontaneous laryngeal reinnervation after recurrent laryngeal or vagus nerve injury. Ann Otol Rhinol Laryngol. 2007;116(1):57–65. https://doi.org/10.1177/000348940711600110.
Wang B, Yuan J, Xu J, Xie J, Wang G, Dong P. Neurotrophin expression and laryngeal muscle pathophysiology following recurrent laryngeal nerve transection. Mol Med Rep. 2016;13(2):1234–42. https://doi.org/10.3892/mmr.2015.4684.
Monaco GN, Brown TJ, Burgette RC, Fargo KN, Akst LM, Jones KJ, Foecking EM. Electrical stimulation and testosterone enhance recovery from recurrent laryngeal nerve crush. Restor Neurol Neurosci. 2015;33(4):571–8. https://doi.org/10.3233/RNN-130334.
Sharma N, Moeller CW, Marzo SJ, Jones KJ, Foecking EM. Combinatorial treatments enhance recovery following facial nerve crush. Laryngoscope. 2010;120(8):1523–30. https://doi.org/10.1002/lary.20997.
Foecking EM, Fargo KN, Coughlin LM, Kim JT, Marzo SJ, Jones KJ. Single session of brief electrical stimulation immediately following crush injury enhances functional recovery of rat facial nerve. J Rehabil Res Dev. 2012;49(3):451–8.
Lever TE, Braun SM, Brooks RT, Harris RA, Littrell LL, Neff RM, Hinkel CJ, Allen MJ, Ulsas MA. Adapting human videofluoroscopic swallow study methods to detect and characterize dysphagia in murine disease models. J Vis Exp. 2015;97:e52319. https://doi.org/10.3791/52319.
Thomas LB, Stemple JC, Andreatta RD, Andrade FH. Establishing a new animal model for the study of laryngeal biology and disease: an anatomic study of the mouse larynx. J Speech Lang Hear Res. 2009;52(3):802–11. https://doi.org/10.1044/1092-4388(2008/08-0087).
Kingham PJ, Birchall MA, Burt R, Jones A, Terenghi G. Reinnervation of laryngeal muscles: a study of changes in myosin heavy chain expression. Muscle Nerve. 2005;32(6):761–6. https://doi.org/10.1002/mus.20409.
Shock LA, Gallemore BC, Hinkel CJ, Szewczyk MM, Hopewell BL, Allen MJ, Thombs LA, Lever TE. Improving the utility of laryngeal adductor reflex testing: a translational tale of mice and men. Otolaryngol Head Neck Surg. 2015;153(1):94–101. https://doi.org/10.1177/0194599815578103.
Lever T, Brooks R, Thombs L, Littrell L, Harris R, Allen M, Kadosh M, Robbins K. Videofluoroscopic validation of a translational murine model of presbyphagia. Dysphagia. 2015;30(3):328–42.
Björck G, Margolin G, Måbäck GM, Persson JK, Mattsson P, Hydman J. New animal model for assessment of functional laryngeal motor innervation. Ann Otol Rhinol Laryngol. 2012;121(10):695–9. https://doi.org/10.1177/000348941212101013.
Paskhover B, Wadie M, Sasaki CT. Thyroarytenoid cross-innervation by the external branch of the superior laryngeal nerve in the porcine model. Laryngoscope. 2015;125(1):177–9. https://doi.org/10.1002/lary.24888.
Lever TE, Simon E, Cox KT, Capra NF, O’Brien KF, Hough MS, Murashov AK. A mouse model of pharyngeal dysphagia in amyotrophic lateral sclerosis. Dysphagia. 2010;25(2):112–26. https://doi.org/10.1007/s00455-009-9232-1.
Sang Q, Goyal RK. Swallowing reflex and brain stem neurons activated by superior laryngeal nerve stimulation in the mouse. Am J Physiol Gastrointest Liver Physiol. 2001;280(2):G191–200.
Bridge PM, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA, Hertl C. Nerve crush injuries—a model for axonotmesis. Exp Neurol. 1994;127(2):284–90. https://doi.org/10.1006/exnr.1994.1104.
Hetzler LE, Sharma N, Tanzer L, Wurster RD, Leonetti J, Marzo SJ, Jones KJ, Foecking EM. Accelerating functional recovery after rat facial nerve injury: effects of gonadal steroids and electrical stimulation. Otolaryngol Head Neck Surg. 2008;139(1):62–7. https://doi.org/10.1016/j.otohns.2008.02.006.
Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20(7):2602–8.
Gordon T, Udina E, Verge VM, de Chaves EI. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Mot Control. 2009;13(4):412–41.
Hernández-Morato I, Valderrama-Canales FJ, Berdugo G, Arias G, McHanwell S, Sañudo J, Vázquez T, Pascual-Font A. Reorganization of laryngeal motoneurons after crush injury in the recurrent laryngeal nerve of the rat. J Anat. 2013;222(4):451–61. https://doi.org/10.1111/joa.12031.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–8.
Mu L, Sanders I. Sensory nerve supply of the human oro- and laryngopharynx: a preliminary study. Anat Rec. 2000;258(4):406–20. https://doi.org/10.1002/(sici)1097-0185(20000401)258:4%3c406:aid-ar9%3e3.0.co;2-5.
Mu L, Sanders I. Sihler’s whole mount nerve staining technique: a review. Biotech Histochem. 2010;85(1):19–42. https://doi.org/10.3109/10520290903048384.
Su WF, Liu SC, Wang SD, Su WY, Ma KH, Huang TT. Nerve branches to the posterior cricoarytenoid muscle may complicate the laryngeal reinnervation procedure. Laryngoscope. 2015;125(2):419–23. https://doi.org/10.1002/lary.24944.
Mu L, Sanders I. The human cricothyroid muscle: three muscle bellies and their innervation patterns. J Voice. 2009;23(1):21–8. https://doi.org/10.1016/j.jvoice.2007.08.001.
Lever TE, Gorsek A, Cox KT, O’Brien KF, Capra NF, Hough MS, Murashov AK. An animal model of oral dysphagia in amyotrophic lateral sclerosis. Dysphagia. 2009;24(2):180–95. https://doi.org/10.1007/s00455-008-9190-z.
Lee SH, Koh KS, Song WC. Oblique thyroarytenoid muscle in humans: an independent muscle or an accessory belly? Laryngoscope. 2018;128(7):1634–8. https://doi.org/10.1002/lary.27090.
Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2015. https://doi.org/10.1016/j.lfs.2015.10.025.
Gould FDH, Yglesias B, Ohlemacher J, German RZ. Pre-pharyngeal swallow effects of recurrent laryngeal nerve lesion on bolus shape and airway protection in an infant pig model. Dysphagia. 2017;32(3):362–73. https://doi.org/10.1007/s00455-016-9762-2.
Duffy JR. Motor speech disorders: substrates, differential diagnosis, and management. Amsterdam: Elsevier; 2005.
DeLozier KR, Gould FDH, Ohlemacher J, Thexton AJ, German RZ. Impact of recurrent laryngeal nerve lesion on oropharyngeal muscle activity and sensorimotor integration in an infant pig model. J Appl Physiol. 2018;125(1):159–66. https://doi.org/10.1152/japplphysiol.00963.2017.
Gong L, Zhu Y, Xu X, Li H, Guo W, Zhao Q, Yao D. The effects of claudin 14 during early Wallerian degeneration after sciatic nerve injury. Neural Regen Res. 2014;9(24):2151–8. https://doi.org/10.4103/1673-5374.147946.
Wu D, Murashov AK. Molecular mechanisms of peripheral nerve regeneration: emerging roles of microRNAs. Front Physiol. 2013;4:55. https://doi.org/10.3389/fphys.2013.00055.
Scerrino G, Tudisca C, Bonventre S, Raspanti C, Picone D, Porrello C, Paladino NC, Vernuccio F, Cupido F, Cocorullo G, Lo Re G, Gulotta G. Swallowing disorders after thyroidectomy: what we know and where we are. A systematic review. Int J Surg. 2017;41(Suppl 1):S94–102. https://doi.org/10.1016/j.ijsu.2017.03.078.
Shriver MF, Lewis DJ, Kshettry VR, Rosenbaum BP, Benzel EC, Mroz TE. Dysphagia rates after anterior cervical diskectomy and fusion: a systematic review and meta-analysis. Glob Spine J. 2017;7(1):95–103. https://doi.org/10.1055/s-0036-1583944.
Logemann JA, Larsen K. Oropharyngeal dysphagia: pathophysiology and diagnosis for the anniversary issue of diseases of the Esophagus. Dis Esophagus. 2012;25(4):299–304. https://doi.org/10.1111/j.1442-2050.2011.01210.x.
Anderson KK, Arnold PM. Oropharyngeal Dysphagia after anterior cervical spine surgery: a review. Glob Spine J. 2013;3(4):273–86. https://doi.org/10.1055/s-0033-1354253.
Yue WM, Brodner W, Highland TR. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year follow-up study. Eur Spine J. 2005;14(7):677–82. https://doi.org/10.1007/s00586-004-0849-3.
Henry BM, Vikse J, Graves MJ, Sanna S, Sanna B, Tomaszewska IM, Tubbs RS, Tomaszewski KA. Extralaryngeal branching of the recurrent laryngeal nerve: a meta-analysis of 28,387 nerves. Langenbeck’s Arch Surg. 2016;401(7):913–23. https://doi.org/10.1007/s00423-016-1455-7.
Cetin F, Gurleyik E, Dogan S. Morphology and functional anatomy of the recurrent laryngeal nerve with extralaryngeal terminal bifurcation. Anat Res Int. 2016;2016:9503170. https://doi.org/10.1155/2016/9503170.
Wong JN, Olson JL, Morhart MJ, Chan KM. Electrical stimulation enhances sensory recovery: a randomized controlled trial. Ann Neurol. 2015;77(6):996–1006. https://doi.org/10.1002/ana.24397.
Schneider R, Randolph GW, Barczynski M, Dionigi G, Wu CW, Chiang FY, Machens A, Kamani D, Dralle H. Continuous intraoperative neural monitoring of the recurrent nerves in thyroid surgery: a quantum leap in technology. Gland Surg. 2016;5(6):607–16. https://doi.org/10.21037/gs.2016.11.10.
Anuwong A, Lavazza M, Kim HY, Wu CW, Rausei S, Pappalardo V, Ferrari CC, Inversini D, Leotta A, Biondi A, Chiang FY, Dionigi G. Recurrent laryngeal nerve management in thyroid surgery: consequences of routine visualization, application of intermittent, standardized and continuous nerve monitoring. Updates Surg. 2016;68(4):331–41. https://doi.org/10.1007/s13304-016-0393-9.
Deniwar A, Bhatia P, Kandil E. Electrophysiological neuromonitoring of the laryngeal nerves in thyroid and parathyroid surgery: a review. World J Exp Med. 2015;5(2):120–3. https://doi.org/10.5493/wjem.v5.i2.120.
Barczyński M, Stopa M, Konturek A, Nowak W. The overwhelming majority but not all motor fibers of the bifid recurrent laryngeal nerve are located in the anterior extralaryngeal branch. World J Surg. 2016;40(3):629–35. https://doi.org/10.1007/s00268-015-3257-4.
Miyauchi A, Masuoka H, Nakayama A, Higashiyama T. Innervation of the cricothyroid muscle by extralaryngeal branches of the recurrent laryngeal nerve. Laryngoscope. 2016;126(5):1157–62. https://doi.org/10.1002/lary.25691.
Tang WJ, Sun SQ, Wang XL, Sun YX, Huang HX. An applied anatomical study on the recurrent laryngeal nerve and inferior thyroid artery. Surg Radiol Anat. 2012;34(4):325–32. https://doi.org/10.1007/s00276-011-0905-8.
Chiang FY, Lu IC, Chen HC, Chen HY, Tsai CJ, Hsiao PJ, Lee KW, Wu CW. Anatomical variations of recurrent laryngeal nerve during thyroid surgery: how to identify and handle the variations with intraoperative neuromonitoring. Kaohsiung J Med Sci. 2010;26(11):575–83. https://doi.org/10.1016/S1607-551X(10)70089-9.
Haney MM, Hamad A, Leary E, Bunyak F, Lever TE. Automated quantification of vocal fold motion in a recurrent laryngeal nerve injury mouse model. Laryngoscope. 2018. https://doi.org/10.1002/lary.27609.
Kelly AM, Leslie P, Beale T, Payten C, Drinnan MJ. Fibreoptic endoscopic evaluation of swallowing and videofluoroscopy: does examination type influence perception of pharyngeal residue severity? Clin Otolaryngol. 2006;31(5):425–32. https://doi.org/10.1111/j.1749-4486.2006.01292.x.
Kelly AM, Drinnan MJ, Leslie P. Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare? Laryngoscope. 2007;117(10):1723–7. https://doi.org/10.1097/MLG.0b013e318123ee6a.
Scott A, Perry A, Bench J. A study of interrater reliability when using videofluoroscopy as an assessment of swallowing. Dysphagia. 1998;13(4):223–7. https://doi.org/10.1007/PL00009576.
Kuhlemeier KV, Yates P, Palmer JB. Intra- and interrater variation in the evaluation of videofluorographic swallowing studies. Dysphagia. 1998;13(3):142–7. https://doi.org/10.1007/PL00009564.
Lee JW, Randall DR, Evangelista LM, Kuhn MA, Belafsky PC. Subjective assessment of videofluoroscopic swallow studies. Otolaryngol Head Neck Surg. 2017;156(5):901–5. https://doi.org/10.1177/0194599817691276.
Tohara H, Nakane A, Murata S, Mikushi S, Ouchi Y, Wakasugi Y, Takashima M, Chiba Y, Uematsu H. Inter- and intra-rater reliability in fibroptic endoscopic evaluation of swallowing. J Oral Rehabil. 2010;37(12):884–91. https://doi.org/10.1111/j.1365-2842.2010.02116.x.
Acknowledgements
We graciously thank Dr. Christina Goldstein for providing translational surgical insight from an orthopedic perspective. Alex Hackworth and Connor (Beau) Burkett assisted with post-mortem laryngeal nerve dissections in mice. Marc Trautmann performed microtome sectioning of paraffin-embedded murine laryngeal specimens. We also acknowledge Roderic Schlotzhauer (MU Physics Machine Shop) for contribution to design and construction of the custom surgical table and laryngoscope tips essential to this study.
Funding
This study was partially funded by an NIH-T32 Grant (#2T32OD011126-38), which provided a postdoctoral stipend and research support for Megan Haney, DVM (sponsor: Lever).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no other potential conflicts of interest.
Research Involving Human Participants and/or Animals
No human participants were included in this study, only animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mok, A., Allen, J., Haney, M.M. et al. A Surgical Mouse Model for Advancing Laryngeal Nerve Regeneration Strategies. Dysphagia 35, 419–437 (2020). https://doi.org/10.1007/s00455-019-10045-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-019-10045-6