Abstract
Isosteviol, a prodrug used to be obtained via Wagner–Meerwein rearrangement from steviol with low yield and long reaction time. Herein, an in-situ separation-coupling-reaction is presented to prepare isosteviol from the natural sweetener stevioside. Simply with in-situ water-washing, the product containing 92.98% purity of isosteviol was obtained with a stevioside conversion of 97.23% from a packet bed reactor without further separation. Within the assayed inorganic acid, organic acids and acidic ionic liquids, the acidic ion-exchange resins provided higher product specificity towards isosteviol. Furthermore, comparing to 5-Fluorouracil, the product presented similar and even stronger inhibition on proliferation of the assayed human cancer cells in a time and dose-dependence by causing cell phase arrest. Isosteviol treatment caused G1 arrest on SGC-7901, HCT-8 and HCT-116 cells, S arrest on HepG2, Huh-7 and HepG3B cells, and G2 arrest on MGC-803 cells, respectively.
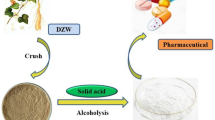
Graphic abstract
Reaction coupling separation for isosteviol production catalyzed by acidic ion-exchange resin.








Similar content being viewed by others
References
Dolder F, Lichti H, Mosettig E, Quitt P (1960) The Structure and Stereochemistry of Steviol and Isosteviol. J Am Chem Soc 82:246–247
Mosettig E, Beglinger U, Dolder F, Lichti H, Quitt P, Waters JA (1963) The absolute configuration of steviol and isosteviol. J Am Chem Soc 85:2305–2309
Sharipova RR, Andreeva OV, Garifullin BF, Strobykina IY, Strobykina AS, Voloshina AD, Kravchenko MA, Kataev VE (2018) Synthesis and antimicrobial and antituberculosis activity of the first conjugates of the diterpenoid isosteviol and D-arabinofuranose. Chem Nat Compd 54:92–97
Liu Y, Wang TT, Ling Y, Bao N, Shi W, Chen L, Sun JB (2017) Design, synthesis and cytotoxic evaluation of nitric oxide-releasing derivatives of isosteviol. Chem Biol Drug Des 90:473–477
Luan T, Cao LH, Deng H, Shen QK, Tian YS, Quan ZS (2019) Design and synthesis of C-19 isosteviol derivatives as potent and highly selective antiproliferative agents. Molecules 24:121
Ullah A, Munir S, Mabkhot Y, Badshah SL (2019) Bioactivity profile of the diterpene isosteviol and its derivatives. Molecules 24:678
Garifullin BF, Strobykina IY, Khabibulina LR, Sapunova AS, Voloshina AD, Sharipova RR, Khairutdinov BI, Zuev YF, Kataev VE (2019) Synthesis and cytotoxicity of the conjugates of diterpenoid isosteviol and N-acetyl-D-glucosamine. Nat Prod Res. https://doi.org/10.1080/14786419.2019.1650355
Liu CJ, Zhang T, Yu SL, Dai XJ, Wu Y, Tao JC (2017) Synthesis, cytotoxic activity, and 2D-and 3D-QSAR studies of 19-carboxyl-modified novel isosteviol derivatives as potential anticancer agents. Chem Biol Drug Des 89:870–887
Malki A, El-Sharkawy A, El Syaed M, Bergmeier S (2017) Antitumor activities of the novel isosteviol derivative 10C against liver cancer. Anticancer Res 37:1591–1601
Meninno S (2019) Valorization of waste: sustainable organocatalysts from renewable resources. Chemsuschem. https://doi.org/10.1002/cssc.201902500
Mathur S, Bulchandani N, Parihar S, Shekhawat GS (2017) Critical review on steviol glycosides: Pharmacological, toxicological and therapeutic aspects of high potency zero caloric sweetener. Int J Pharmacol 13:916–928
Ruiz-Ruiz JC, Moguel-Ordonez YB, Segura-Campos MR (2017) Biological activity of Stevia rebaudiana Bertoni and their relationship to health. Crit Rev Food Sci 57:2680–2690
Carrera-Lanestosa A, Moguel-Ordonez Y, Segura-Campos M (2017) Stevia rebaudiana Bertoni: a natural alternative for treating diseases associated with metabolic syndrome. J Med Food 20:933–943
Zhang H, Sun X, Xie Y, Tian F, Hu H, Tan W (2018) Isosteviol sodium inhibits astrogliosis after cerebral ischemia/ reperfusion injury in rats. Biol Pharm Bull 41:575–584
Avent AG, Hanson JR, Hitchcock PB, De Oliveira BH (1990) The influence of a 15-hydroxy group on the rearrangement reactions of steviol and its 16,17-epoxide. J Chem Soc Perkin Trans 1:2661–2665
Khaibullin RN, Strobykina IY, Kataev VE, Lodochnikova OA, Gubaidullin AT, Musin RZ (2009) New synthesis of diterpenoid (16S)-dihydrosteviol. Russ J Gen Chem 79:967–971
Li HP, Dong CM, Hou YM, Liu HM (2009) Synthesis of 17-OH-Isosteviol. Chem Res Chin Univ 25:116–117
Lohoelter C, Weckbecker M, Waldvogel SR (2013) (–)-Isosteviol as a versatile ex-chiral-pool building block for organic chemistry. Eur J. Org Chem 2013:5539–5554
Perera WH, Docampo ML, Wiggers FT, Hufford CD, Fronczek FR, Avula B, Khan IA, McChesney JD (2018) Endocyclic double bond isomers and by-products from rebaudioside A and stevioside formed under acid conditions. Phytochem Lett 25:163–170
Wan HD, He GZ, Zhang HJ (2019) Isosteviol preparation and inclusion complexation of it with -cyclodextrin. J Incl Phenom Macro 94:65–73
Cherney EC, Green JC, Baran PS (2013) Synthesis of ent-kaurane and beyerane diterpenoids by controlled fragmentations of overbred intermediates. Angew Chem Int Ed 52:9019–9022
Milagre HMS, Martins LR, Takahashi JA (2009) Novel agents for enzymatic and fungal hydrolysis of stevioside. Brazilian Journal of Microbiology 40:367–372
Víctor-Ortega MD, Ochando-Pulido JM, Martínez-Ferez A (2016) Iron removal and reuse from Fenton-like pretreated olive mill wastewater with novel strong-acid cation exchange resin fixed-bed column. J Ind Eng Chem 36:298–305
Chen JM, Xia YM, Wan HD, Wang HJ, Liu X (2014) A complete specific cleavage of glucosyl and ester linkages of stevioside for preparing steviol with a β-galactosidase from Sulfolobus solfataricus. J Mol Catal B Enzym 105:126–131
Chen JM, Ding L, Sui XC, Xia YM, Wan HD, Lu T (2016) Production of a bioactive sweetener steviolbioside via specific hydrolyzing ester linkage of stevioside with a beta-galactosidase. Food Chem 196:155–160
Chen J-M, Zhang J, Xia Y-M, Wang X-X, Li J (2018) The natural sweetener metabolite steviol inhibits the proliferation of human osteosarcoma U2OS cell line. Oncol Lett 15:5250–5256
Ye J, Pei X, Cui H, Yu Z, Lee H, Wang J, Wang X, Sun L, He H, Yang VC (2018) Cellular uptake mechanism and comparative in vitro cytotoxicity studies of monomeric LMWP-siRNA conjugate. J Ind Eng Chem 63:103–111
Oliveira BH, Strapasson RA (1996) Biotransformation of isosteviol by Fusarium verticilloides. Phytochemistry 43:393–395
Strobykina IY, Belenok MG, Semenova MN, Semenov VV, Babaev VM, Rizvanov IK, Mironov VF, Kataev VE (2015) Triphenylphosphonium cations of the diterpenoid isosteviol: synthesis and antimitotic activity in a sea urchin embryo model. J Nat Prod 78:1300–1308
Mizushina Y, Akihisa T, Ukiya M, Hamasaki Y, Murakami-Nakai C, Kuriyama I, Takeuchi T, Sugawara F, Yoshida H (2005) Structural analysis of isosteviol and related compounds as DNA polymerase and DNA topoisomerase inhibitors. Life Sci 77:2127–2140
Momtazi-Borojeni AA, Esmaeili SA, Abdollahi E, Sahebkar A (2017) A review on the pharmacology and toxicology of steviol glycosides extracted from stevia rebaudiana. Curr Pharm Design 23:1616–1622
Takasaki M, Konoshima T, Kozuka M, Tokuda H, Takayasu J, Nishino H, Miyakoshi M, Mizutani K, Lee KH (2009) Cancer preventive agents. Part 8: chemopreventive effects of stevioside and related compounds. Bioorgan Med Chem 17:600–605
Bazotte RB, Lonardoni MTC, Alvarez M, Gaeti WP, Amado CAB (1986) Determination of the lethal dose (LD50) for the lethal isosteviol in laboratory animals. Dev Med Child Neurol 18:103–116
Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama J-i, Yamamura T, Hashimoto-Tamaoki T (2001) Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Can Res 61:1029–1037
Acknowledgements
Financial support from National Natural Science Foundation of China (31772017, 31371837), the national first-class discipline program of Light Industry Technology and Engineering (LITE2018-03), and the project of outstanding scientific & technological innovation group of Jiangsu Province are gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, X., Zhou, Z., Zhang, Z. et al. Reaction coupling separation for isosteviol production from stevioside catalyzed by acidic ion-exchange resin. Bioprocess Biosyst Eng 44, 151–159 (2021). https://doi.org/10.1007/s00449-020-02431-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02431-4