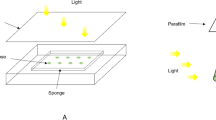
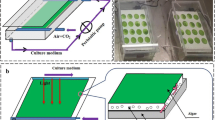
Abstract
The influences of urea, nitrate and glycine with four concentration levels on attached culture of Nannochloropsis oculata were investigated. The organic nitrogen source glycine was effective on improving not only adhesion biomass productivity but also adhesion rate. The maximum adhesion biomass productivity of 15.76 ± 0.52 g m−2 day−1 with adhesion rate of 76.67 ± 0.42 % was achieved with 18 mM glycine. To increase the lipid production, three lipid enhancing strategies were conducted afterwards, including nitrogen starvation, high light, and the combination of nitrogen starvation and high light. In nitrogen starvation situation, although the lipid content was greatly increased, the adhesion biomass productivity dropped probably due to the low cell viability. Increasing light intensity was effective on enhancing both adhesion biomass productivity and lipid content. The results indicated that nitrogen starvation was effective on improving both lipid content and adhesion rate when high light was applied. The maximum lipid yield of 4.32 ± 0.14 g m−2 day−1 with adhesion biomass productivity of 21.32 ± 0.65 g m−2 day−1, adhesion rate of 86.81 ± 0.10 % and lipid content of 20.24 ± 0.06 % was achieved with the combination strategy.


Similar content being viewed by others
References
Kawata M, Nanba M, Matsukawa R, Chihara M, Karube I (1998) Isolation and characterization of a green alga Neochloris sp. for CO2 fixation. Stud Surf Sci Catal 114:637–640
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Stephens E, Ross IL, King Z, Mussgnug JH, Kruse O, Posten C, Borowitzka MA, Hankamer B (2010) An economic and technical evaluation of microalgal biofuels. Nat Biotechnol 28(1):26–128
Moheimani NR, Borowitzka MA (2006) The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J Appl Phycol 18:703–712
Carlozzi P (2003) Dilution of solar radiation through “culture lamination” in photobioreactor rows facing south north: a way to improve the efficiency of light utilization. Biotechnol Bioeng 81:305–315
Johnson M, Wen Z (2010) Development of an attached microalgal growth system for biofuel production. Appl Microbiol Biotechnol 85:525–534
Gross M, Henry W, Michael C, Wen Z (2013) Development of a rotating algal biofilm growth system for attached microalgae growth with in-situ biomass harvest. Bioresour Technol 150:195–201
Christenson L, Sims R (2012) Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotech Bioeng 109:1674–1684
Schnurr P, Espie G, Allen G (2013) Algae biofilm growth and the potential to stimulate lipid accumulation through nutrient starvation. Bioresour Technol 136:337–344
Courchesne NMD, Parisien A, Wang B et al (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141:31–41
Illman AM, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym Microb Technol 27:631–635
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252
Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak MN (2008) Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J Appl Phycol 20:245–251
Shen Y, Yuan W, Pei Z, Mao E (2010) Heterotrophic culture of Chlorella protothecoides in various nitrogen sources for lipid production. Appl Biochem Biotechnol 160:1674–1684
Guillard R, Ryther JH (1962) Studies of marine planktonic diatoms. I.Cyclotella nana Husted and Detonula confervacea (Cleve) Gran(“F” medium). Can J Microbiol 8:229–239
Shen Y, Chen W, Xu X, Zhao Y (2013) Multi-level photobioreactor for attached microalgal cultivation. Pending Chinese Patent 201310336140.1, 22 Aug 2013
Shen Y, Xu X, Zhao Y, et al. (2013) Influence of algae species, substrata and culture conditions on attached microalgal culture. Bioprocess Biosyst Eng. doi:10.1007/s00449-013-1011-6
Li Y, Zhou W, Hu B et al (2012) Effect of light intensity on algal biomass accumulation and biodiesel production for mixotrophic strains Chlorella kessleri and Chlorella protothecoide cultivated in highly concentrated municipal wastewater. Biotechnol Bioeng 109:2222–2229
Bezerra RP, Matsudo MC, Sato S et al (2012) Effects of photobioreactor configuration, nitrogen source and light intensity on the fed-batch cultivation of Arthrospira (Spirulina) platensis. Bioenergetic aspects. Biomass Bioeng 37:309–317
Liu T, Wang J, Hu Q et al (2012) Attached cultivation technology of microalgae for efficient biomass feedstock production. Bioresour Technol 127:216–222
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals 15:377–390
Fierro S, del Pilar Sanchez-Saavedra M, Copalcua C (2008) Nitrate and phosphate removal by chitosan immobilized Scenedesmus. Bioresour Technol 99:1274–1279
Molina Grima EM, Belarbi EH, Fernandez FGA, Medina AR, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515
Sekar R, Venugopalan VP, Satpathy KK, Nair KVK, Rao VNR (2004) Laboratory studies on adhesion of microalgae to hard substrates. Biomed Life Sci 173(2):109–116
Bender DA, Bender AE (1999) Benders’ dictionary of nutrition and food technology, 7th edn. CRC Press, Boca Raton
Shen Y, Yuan W, Pei ZJ et al (2009) Microalgae mass production methods. Transactions of the ASABE 52:1275–1287
Chen X, Goh QY, Tan W et al (2011) Lumostatic strategy for microalgae cultivation utilizing image analysis and chlorophyllacontent as design parameters. Bioresour Technol 102:6005–6012
Ruffing AM, Chen RR (2012) Transcriptome profiling of a curdlan-producing Agrobacterium reveals conserved regulatory mechanisms of exopolysaccharide biosynthesis. Microb Cell Fact 11:1–13
Bragadeeswaran S, Jeevapriya R, Prabhu K et al (2011) Exopolysaccharide production by Bacillus cereus GU812900, a fouling marine bacterium. Afr J Microbiol Res 5:4124–4132
Stapleton RD Jr, Singh VP (2002) Biotransformations: bioremediation technology for health and environmental protection. Elsevier, Amsterdam
Acknowledgments
This research was financially supported by the Natural Science Foundation of China (Award 51108085), the Natural Science Foundation of Fujian Province (Award No. 2011J05125) and the Program of the Education Department of Fujian Province (Award JA11030).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, Y., Chen, C., Chen, W. et al. Attached culture of Nannochloropsis oculata for lipid production. Bioprocess Biosyst Eng 37, 1743–1748 (2014). https://doi.org/10.1007/s00449-014-1147-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1147-z