Abstract
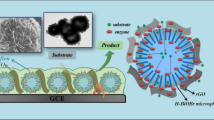
ZnO hollow spheres were firstly prepared. A new type of amperometric hydrogen peroxide biosensor was fabricated by entrapping Hemoglobin (Hb) through the ZnO hollow spheres (ZHS) nanoparticles. The composition morphology and size were studied by transmission electron microscopy. The surface topography of the prepared films was imaged by atomic force microscope (AFM). Several techniques, including UV–vis absorption spectroscopy, cyclic voltammetry, chronoamperometry were employed to characterize the performance of the biosensor. The results indicated that the ZHS nanoparticles had enhanced the performance of the hydrogen peroxide sensors. The electrochemical parameters of Hb in the ZHS were calculated by the results of the electron-transfer coefficient (α) and the apparent heterogeneous electron-transfer rate constant K s as 0.5 and 3.1 s−1, respectively. The resulting biosensors showed a wide linear range from 2.1 × 10−6 to 5.18 × 10−3 M, with a low detection limit of 7.0 × 10−7 M (S/N = 3) under optimized experimental conditions. The results demonstrated that the ZHS matrix may improve the protein loading with the retention of bioactivity and greatly promote the direct electron transfer, which can be attributed to its unique morphology, high specific surface area, and biocompatibility. The biosensor obtained from this study possesses high sensitivity, good reproducibility, and long-term stability.











Similar content being viewed by others
References
Armstrong FA (2002) Insights from protein film voltammetry into mechanisms of complex biological electron-transfer reactions. J Chem Soc Dalton Trans 5:661–671
Willner I, Katz E (2000) Integration of layered redox-proteins and conductive supports for bioelectronic applications. Angew Chem Int Ed 39:1180–1218
Hill HAO (1996) The development of bioelectrochemistry. Coord Chem Rev 151:115–123
Yu JJ, Zhao T, Zhao FQ et al (2008) Direct electron transfer of hemoglobin immobilized in a mesocellular siliceous foams supported room temperature ionic liquid matrix and the electrocatalytic reduction of H2O2. Electrochim Acta 53:5760–5765
He XY, Zhu L (2006) Direct electrochemistry of hemoglobin in cetylpyridinium bromide film: redox thermodynamics and electrocatalysis to nitric oxide. Electrochem Commun 8:615–620
Lu Q, Zhou T, Hu SS (2007) Direct electrochemistry of hemoglobin in PHEA and its catalysis to H2O2. Biosens Bioelectron 22:899–904
He PL, Hu NF, Rusling JF (2004) Driving Forces for layer-by-layer self-assembly of films of SiO2 nanoparticles and heme proteins. Langmuir 20:722–729
Xian YZ, Xian Y, Zhou LH et al (2007) Encapsulation hemoglobin in ordered mesoporous silicas: Influence factors for immobilization and bioelectrochemistry. Electrochem Commun 9:142–148
Bistolas N, Wollenberger U, Jung C et al (2005) Cytochrome P450 biosensors—a review. Biosens Bioelectron 20:2408–2423
Huang H, Hu NF, Zeng Y et al (2002) Electrochemistry and electrocatalysis with heme proteins in chitosan biopolymer films. Anal Biochem 308:141–151
Lu XB, Wen ZH, Li JH (2006) Hydroxyl-containing antimony oxide bromide nanorods combined with chitosan for biosensors. Biomaterials 27:5740–5747
Chen D, Liu JS, Wang P et al (2007) Fabrication of monodisperse zirconia-coated core–shell and hollow spheres in mixed solvents. Colloids Surf A: Physicochem Eng Aspects 302:461–466
Graf C, Blaaderen AV (2002) Metallodielectric colloidal core–shell particles for photonic applications. Langmuir 18:524–534
Liu RJ, Li YJ, Zhao H et al (2008) Synthesis and characterization of Al2O3 hollow spheres. Mater Lett 62:2593–2595
Kalele SA, Ashtaputre SS, Hebalkar NY et al (2005) Optical detection of antibody using silica–silver core–shell particles. Chem Phys Lett 404:136–141
Applerot G, Lipovsky A, Dror R et al (2009) Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv Funct Mater 19:842–852
Liu ZM, Liu YL, Yang HF et al (2005) A mediator-free tyrosinase biosensor based on ZnO sol–gel matrix. Electroanalysis 17:1065–1070
Lu XB, Zhang HJ, Ni YW et al (2008) Porous nanosheet-based ZnO microspheres for the construction of direct electrochemical biosensors. Biosens Bioelectron 24:93–98
Li XF, Lv KL, Deng KJ et al (2009) Synthesis and characterization of ZnO and TiO2 hollow spheres with enhanced photoreactivity. Mater Sci Eng B 158:40–47
Agrawal M, Gupta S, Pich A et al (2009) A facile approach to fabrication of ZnO–TiO2 hollow spheres. Chem Mater 21:5343–5348
Deng ZW, Chen M, Gu GX et al (2008) A facile method to fabricate ZnO hollow spheres and their photocatalytic property. J Phys Chem B 11:216–222
Murray RW, Bard AJ (1984) Electroanalytical chemistry, vol 13. Marcel Dekker, New York, pp 191–368
Laviron E (1974) Adsorption autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem 52:355–393
Liu HH, Tian ZQ, Lu ZX et al (2004) Direct electrochemistry and electrocatalysis of heme-proteins entrapped in agarose hydrogel films. Biosens Bioelectron 20:294–304
Laviron E (1979) The use of linear potential sweep voltammetry and of a.c. voltammetry for the study of the surface electrochemical reaction of strongly adsorbed systems and of redox modified electrodes. J Electroanal Chem 100:263–270
Liu YG, Lu CL, Hou WH et al (2008) Direct electron transfer of hemoglobin in layered a-zirconium phosphate with a high thermal stability. Anal Biochem 375:27–34
Sun W, Zhai ZQ, Wang DD et al (2009) Electrochemistry of hemoglobin entrapped in a Nafion/nano-ZnO film on carbon ionic liquid electrode. Bioelectrochemistry 74:295–300
Nassar AE, Zhang Z, Hu N et al (1997) Proton coupled electron transfer from electrodes to myoglobin in ordered biomembrane-like films. J Phys Chem B 101:2224–2231
Huang H, He P, Hu N et al (2003) Electrochemical and electrocatalytic properties of myoglobin and hemoglobin incorporated in carboxymethyl cellulose films. Bioelectrochemistry 61:29–38
Trushina E, Oda R, Landers J et al (1997) Determination of nitrite and nitrate reduction by capillary ion electrophoresis. Electrophoresis 18:1890–1898
Zheng W, Li J, Zheng YF (2008) An amperometric biosensor based on hemoglobin immobilized in poly(ε-caprolactone) film and its application. Biosens Bioelectron 23:1562–1566
Wang Y, Qian WQ, Tan Y et al (2007) Direct electrochemistry and electroanalysis of hemoglobin adsorbed in self-assembled films of gold nanoshells. Talanta 72:1134–1140
Lai GS, Zhang HL, Han DY (2008) A novel hydrogen peroxide biosensor based on hemoglobin immobilized on magnetic chitosan microspheres modified electrode. Sensors Actuators B 129:497–503
Kamin A, Wilson GS (1980) Rotating ringdisk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal Chem 52:1198–1205
Zhao G, Feng JJ, Xu J et al (2005) Direct electrochemistry and electrocatalysis of heme proteins immobilized on self assembled ZrO2 film. Electrochem Commun 7:724–729
Feng JJ, Xu JJ, Chen HY (2005) Direct electron transfer and electrocatalysis of hemoglobin adsorbed onto electrodeposited mesoporous tungsten oxide. Electrochem Commun 8:77–82
Liu MC, Zhao GH, Zhao KJ et al (2009) Direct electrochemistry of hemoglobin at vertically aligned self-doping TiO2 nanotubes: a mediator-free and biomolecule-substantive electrochemical interface. Electrochem Commun 11:1397–1400
Rui Q, Komori KK, Tian Y et al (2010) Electrochemical biosensor for the detection of H2O2 from living cancer cells based on ZnO nanosheets. Anal Chim Acta 670:57–62
Zhang YW, Zhang Y, Wang H et al (2009) An enzyme immobilization platform for biosensor designs of direct electrochemistry using flower-like ZnO crystals and nano-sized gold particles. J Electroanal Chem 627:9–14
Xiang CL, Zou YJ, Sun LX et al (2009) Direct electrochemistry and enhanced electrocatalysis of horseradish peroxidase based on flowerlike ZnO–gold nanoparticle—Nafion nanocomposite. Sens Actuators B 136:158–162
Yang Z, Zong XL, Ye ZZ et al (2010) The application of complex multiple fork like ZnO nanostructures to rapid and ultrahigh sensitive hydrogen peroxide biosensors. Biomaterials 31:7534–7541
Zhao G, Xu JJ, Chen H (2006) Interfacing myoglobin to graphite electrode with an electrodeposited nanoporous ZnO film. Anal Biochem 350:145–150
Kafi AKM, Lee DY, Park SH et al (2007) Amperometric biosensor based on direct electrochemistry of hemoglobin in poly allylamine (PAA) film. Thin Solid Films 515:5179–5183
Chen L, Lu GX (2007) Novel amperometric biosensor based on composite film assembled by polyelectrolyte-surfactant polymer, carbon nanotubes and hemoglobin. Sens. Actuators B 121:423–429
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, C., Xu, J. & Wu, Z. Direct electron transfer and electrochemical study of hemoglobin immobilized in ZnO hollow spheres. Bioprocess Biosyst Eng 34, 931–938 (2011). https://doi.org/10.1007/s00449-011-0544-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0544-9