Abstract
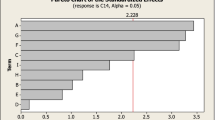
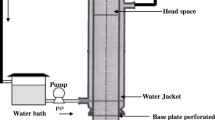
Based on the response surface methodology, an effective microbial system for diosgenin production from enzymatic pretreated Dioscorea zingiberensis tubers with Trichoderma reesei was studied. The fermentation medium was optimized with central composite design (35) depended on Plackett–Burmann design which identified significant impacts of peptone, K2HPO4 and Tween 80 on diosgenin yield. The effects of different fermentation conditions on diosgenin production were also studied. Four parameters, i.e. incubation period, temperature, initial pH and substrate concentration were optimized using 45 central composite design. The highest diosgenin yield of 90.57% was achieved with 2.67% (w/v) of peptone, 0.29% (w/v) of K2HPO4, 0.73% (w/v) of Tween 80 and 9.77% (w/v) of substrate, under the condition of pH 5.8, temperature 30 °C. The idealized incubation time was 6.5 days. After optimization, the product yield increased by 33.70% as compared to 67.74 ± 1.54% of diosgenin yield in not optimized condition. Scale-up fermentation was carried out in a 5.0 l bioreactor, maximum diosgenin yield of 90.17 ± 3.12% was obtained at an aeration of 0.80 vvm and an agitation rate of 300 rpm. The proposed microbial system is clean and effective for diosgenin production and thus more environmentally acceptable than the traditional acid hydrolysis.



Similar content being viewed by others
References
Aradhana M, Rao AC, Kale RK (1992) Diosgenin-A growth stimulator of mammary gland of ovariectomized mouse. Indian J Exp Biol 30:367–370
Cayen MN, Dvornik D (1979) Effect of diosgenin on lipid metabolism in rats. J Lipid Res 20:162–174
Holland RE, Rahman K, Morris AI, Coleman RD, Billington D (1993) Effects of niacin on biliary lipid output in the rat. Biochem Pharmacol 45:43–49
Oncina R, Botía JM, Del Río JA, Ortuño A (2000) Bioproduction of diosgenin in callus cultures of Trigonella foenum-graecum L. Food Chem 70:489–492
Zhang CX, Wang YX, Yang ZH, Xu MH (2006) Chlorine emission and dechlorination in co-firing coal and the residue from hydrochloric acid hydrolysis of Discorea zingiberensis. Fuel 85:2034–2040
Bae EA, Kim NY, Han MJ, Choo MK, Kim DH (2003) Transformation of ginsenoside to compound K (IH-901) by lactic acid bacteria of human intestine. J Microbiol Biotechnol 13:9–14
Huang W, Zhao HZ, Ni JR, Zuo H, Qiu LL, Li H (2008) The best utilization of D. zingiberensis C. H. Wright by an ecofriendly process. Bioresour Technol l99:7407–7411
Wang YX, Liu H, Bao JG, Hong Y, Yang ZH, Zhang CX (2008) The saccharification-membrane retrieval-hydrolysis (SMRH) process: a novel approach for cleaner production of diosgenin derived from Dioscorea zingiberensis. J Cleaner Prod 16:1133–1137
Qian SH, Yuan LH, Yang NY, OuYang PK (2006) Study on steroidal compounds from Dioscorea zingiberensis. Chin Traditional Herb Drugs 29:1174–1176 (in Chinese)
Cheng P, Zhao HZ, Zhao B, Ni JR (2009) Pilot treatment of wastewater from Dioscorea zingiberensis C. H. Wright production by anaerobic digestion combined with a biological aerated filter. Bioresour Technol 12:2918–2925
Zhao HZ, Cheng P, Zhao B, Ni JR (2008) Yellow ginger processing wastewater treatment by a hybrid biological process. Process Biochem 43:1427–1431
Wendy AL (2000) Biotransformations in organic synthesis. Bioresour Technol 74:49–62
Qi SS, Dong YS, Zhao YK, Xiu ZL (2009) Qualitative and quantitative analysis of microbial transformation of steroidal saponins in Dioscorea zingiberensis. Chromatographia 69:865–870
Qi SS, Dong YS, Zhao YK, Xiu ZL (2008) Identification and quantification of steroidal saponins and aglycone during biotransformation of Dioscorea zingiberensis by Aspergillus oryzae. J Biotechnol 136:356–401
Zhao YT, Xu ZL, Dai CC, Tang XL, Xia B, Wang Q (2007) Selection of the microorganism for dioscin hydrolyze. J Chin Med Mater 30:905–909 (in Chinese)
Avishek M, Arun G (2008) Enhanced production of exocellular glucansucrase from Leuconostoc dextranicum NRRL B-1146 using response surface method. Bioresour Technol 99:3685–3691
Sheng J, Chi ZM, Yan KR, Wang XH, Gong F, Li J (2009) Use of response surface methodology for optimizing process parameters for high inulinase production by the marine yeast Cryptococcus aureus G7a in solid-state fermentation and hydrolysis of inulin. Bioprocess Biosyst Eng 32:333–339
Liang LY, Zheng YG, Shen YC (2008) Optimization of β-alanine production from β-aminopropointrile by resting cells of Rhodococcus sp. G20 in a bubble column reactor using response surface methodology. Process Biochem 43:758–764
Jin MJ, Huang H, Xiao AH, Gao Z, Liu X, Peng C (2009) Enhancing arachidonic acid production by Mortierella alpine ME-1 using improved mycelium aging technology. Bioprocess Biosyst Eng 32:117–122
Shefali G, Mukesh K, Krishna KS, Lavanya MN, Ramesh CK (2008) Production and recovery of an alkaline exo-polygalacturonase from Bacillus subtilis RCK under solid-state fermentation using statistical approach. Bioresour Technol l99:937–945
Bera MB, Panesar PS, Panesar R, Singh B (2008) Application of reverse micelle extraction process for amylase recovery using response surface methodology. Bioprocess Biosyst Eng 31:379–384
Lai LST, Pan CC, Tzeng BK (2003) The influence of medium design on lovastatin production and pellet formation with a high-producing mutant of Aspergillus terreus in submerged cultures. Process Biochem 38:1317–1326
Liu BL, Tzeng MY (1998) Optimization of growth medium for the production of spores from Bacillus thuringiensis using response surface methodology. Bioprocess Eng 18:413–418
Little TM, Hills FJ (1978) Agricultural experimentation design and analysis. Wiley, New York, p 170
Mu XQ, Xu Y, Nie Y, Ouyang J, Sun ZH (2005) Candida parapsilosis CCTCC M203011 and the optimization of fermentation medium for stereo inversion of(S)-1-phenyl-1, 2-ethanediol. Process Biochem 40:2345–2350
Doppelbauer R, Esterbauer H, Steiner W, Lafferty RM, Steinmuller H (1987) Lignocellulosic waste for production of cellulase by Trichoderma reesei. Appl Microbial Biotechnol 26:485–494
Wen ZY, Liao W, Chen SL (2005) Production of cellulase by Trichoderma reesei from dairy manure. Bioresour Technol 96:491–499
Kamande GM, Baah J, Cheng KJ, McAllister TA, Shelford JA (2000) Effects of Tween 60 and Tween 80 on protease activity, thiol group reactivity, protein adsorption, and cellulose degradation by rumen microbial enzymes. J Dairy Sci 83:536–542
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Zeng GM, Shi JG, Yuan XZ, Liu J, Zhang ZB, Huang GH (2006) Effects of Tween 80 and rhamnolipid on the extracellular enzymes of Penicillium simplicissimum isolated from compost. Enzyme Microb Technol 39:1451–1456
Wang YX, Lu ZX (2005) Optimization of processing parameters for the mycelial growth and extracellular polysaccharide production by Boletus spp. ACCC 50328. Process Biochem 40:1043–1051
Sternberg D, Mandels GR (1979) Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol 139:761–769
Sheldon JBD, William DM (1996) Bioconversion of forest products industry waste cellulosics to fuel ethanols: a review. Bioresour Technol 55:1–33
Bhattacharyya MS, Singh A, Banerjee UC (2008) Production of carbonyl reductase by Geotrichum candidum in a laboratory scale bioreactor. Bioresour Technol 99:8765–8770
Acknowledgments
This work was supported by the National “11th-5-Year” Plan Project of China under Grant 2006BAB04A14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Ni, J. & Huang, W. Process optimization for the production of diosgenin with Trichoderma reesei . Bioprocess Biosyst Eng 33, 647–655 (2010). https://doi.org/10.1007/s00449-009-0390-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-009-0390-1