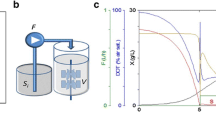
Abstract
A set of mutations in the phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS) was used to create Escherichia coli strains with a reduced uptake rate of glucose. This allows a growth restriction, which is controlled on cellular rather than reactor level, which is typical of the fed-batch cultivation concept. Batch growth of the engineered strains resulted in cell accumulation profiles corresponding to a growth rate of 0.78, 0.38 and 0.25 h−1, respectively. The performance of the mutants in batch cultivation was compared to fed-batch cultivation of the wild type cell using restricted glucose feed to arrive at the corresponding growth profiles. Results show that the acetate production, oxygen consumption and product formation were similar, when a recombinant product was induced from the lacUV5 promoter. Ten times more cells could be produced in batch cultivation using the mutants without the growth detrimental production of acetic acid. This allows high cell density production without the establishment of elaborate fed-batch control equipment. The technique is suggested as a versatile tool in high throughput multiparallel protein production but also for increasing the number of experiments performed during process development while keeping conditions similar to the large-scale fed-batch performance.






Similar content being viewed by others
References
Sandén AM, Boström M, Markland K, Larsson G (2005) Solubility and proteolysis of the Zb-MalE and Zb-MalE31 proteins during overproduction in Escherichia coli. Biotech Bioeng 90:239–247
Boström M, Markland K, Sandén AM, Hedhammar M, Hober S, Larsson G (2005) Effect of substrate feed rate on recombinant protein secretion, degradation and inclusion body formation in Escherichia coli. Appl Microbiol Biotechnol 68:82–90
Sandén AM, Prytz I, Tubulekas I, Förberg C, Le H, Hektor A, Neubauer P, Pragai Z, Harwood C, Ward A, Picon A, Teixeira de Mattos J, Postma P, Farewell A, Nyström T, Reeh S, Pedersen S, Larsson G (2003) Limiting factors in Escherichia coli fed-batch production of recombinant proteins. Biotech Bioeng 81:158–166
Picon A, Teixeira de Mattos MJ, Postma PW (2005) Reducing the glucose uptake rate in Escherichia coli affects growth rate but not protein production. Biotech Bioeng 90:191–200
Linn T, St.Pierre R (1990) Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J Bacteriol 172:1077–1084
Rose R (1998) The nucleotide sequence of pACYC184. Nucl Acid Res 16:355
Larsson G, Törnkvist M (1996) Rapid sampling, cell inactivation and evaluation of low extracellular glucose concentrations during fed-batch cultivation. J Biotechnol 49:69–82
Ferenci T (1996) Adaptation to lift at micromolar nutrient levels: the regulation of Eschrichia coli glucose transport by endoinduction and cAMP. FEMS Microbiol Rev 18:301–317
Doelle H, Ewings K, Hollywood N (1982) Regulation of glucose metabolism in bacterial systems. Adv Biochem Eng 23:1–35
Meyer H-P, Leist C, Fiechter A (1984) Acetate formation in continuous culture of Escherichia coli K12 D1 on defined and complex media. J Biotechnol 1:355–358
Gosset G (2005) Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate:sugar phosphotransferase system. Microb Cell Fact 4:14
Curtis S, Epstein W (1975) Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol 122:1189–1199
Duetz W, Rüedi L, Hermann R, O’Connor K, Büchs J, Witholt B (2000) Methods for intense aeration, growth, storage, and replication of bacterial strains in microtiter plates. Appl Env Micro 66:2641–2646
John GT, Kliman I, Wittman C, Heinzle E (2003) Integrated optical sensing of dissolved oxygen in microtitre plates: a novel tool for microbial cultivation. Biotech Bioeng 81:829–836
Jensen E, Carlsen S (1990) Production of recombinant human growth hormone in Escherichia coli: expression of different precursors and physiological effects of glucose, acetate and salts. Biotech Bioeng 36:1–11
Chou C, Bennett G, San K (1994) Effect of modified glucose uptake using genetic engineering techniques on high-level recombinant protein in Escherichia coli dense cultures. Biotechnol Bioeng 44:952–960
Kimata K, Inada T, Tagami H, Aiba H (1998) A global repressor (Mlc) is involved in glucose induction of the ptsG gene encoding major glucose transporter in Escherichia coli. Mol Microbiol 29:1509–1519
De Anda R, Lara A, Hernandez V, Hernandez-Montalvo V, Gosset G, Bolivar F, Ramirez O (2006) Replacement of the glucose phosphotransferase transport system by galactose permease reduces acetate accumulation and improves process performance of Escherichia coli for recombinant protein production without impairment of growth rate. Metabolic Eng 8:281–290
Cho S, Shin D, Ji GE, Heu S, Ryu S (2005) High-level recombinant protein production by overexpression of Mlc in Escherichia coli. J Biotechnol 119:197–203
Boström M, Larsson G (2004) Process design for recombinant protein production based on the promoter, PmalK. Appl Microbiol Biotechnol 66:200–208
Ryan W, Collier P, Loredo L, Pope J, Sachdev R (1996) Growth kinetics of Escherichia coli and expresiion of a recombinant protein and its isoforms under heat shock conditions. Biotechnol Prog 12:596–601
Plumbridge J (1998) Control of the expression of the manXYZ operon in Escherichia coli: Mlc is a negative regulator of the mannose PTS. Mol Microbiol 27:369–380
Plumbridge J (1999) Expression of the phosphotransferase system both mediates and is mediated by Mlc regulation in Escherichia coli. Mol Microbiol 33:260–273
Acknowledgments
This work was sponsored by the Swedish Centre for Bioprocess Technology, CBioPT, which is gratefully acknowledged. We thank the Lundberg laboratory at the University of Gothenburg (Prof. Thomas Nyström and Dr Anne Farewell) and the University of Amsterdam (Profs. Peter Postma and Joost Teixeira de Mattos) for kindly supplying the strains and plasmids used in this work which were constructed in a joint EU Framework IV project: BIO4-CT98-0167.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bäcklund, E., Markland, K. & Larsson, G. Cell engineering of Escherichia coli allows high cell density accumulation without fed-batch process control. Bioprocess Biosyst Eng 31, 11–20 (2008). https://doi.org/10.1007/s00449-007-0144-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-007-0144-x