Abstract
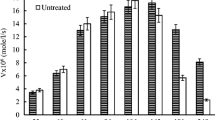
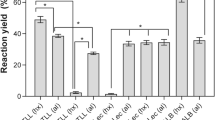
The influence of polyhydric alcohols (sorbitol, xylitol, erythritol, glycerol) on the thermal stability of Rhizomucor miehei lipase has been studied at high hydrostatic pressure (up to 500 MPa). In the absence of additives, a protective effect (PE) (the ratio between the residual activities determined at 480 MPa for the enzyme in the presence or absence of polyhydric alcohols) of low-applied pressures (from 50 MPa to 350 MPa) against thermal deactivations (at 50°C and 55°C) has been noticed. In the presence of additives, a strong correlation between PE and the total hydroxyl group concentration has been obtained, for the first time, under treatments of combining denaturing temperatures and high hydrostatic pressures. This relationship does not seem to be dependent on the nature polyhydric alcohols as the same effect could be observed with 1 M sorbitol and 2 M glycerol. This PE, against thermal and high pressure combined lipase deactivation, increases with polyhydric alcohol concentrations, and when temperature increases from 25°C to 55°C.




Similar content being viewed by others
Abbreviations
- C n :
-
n carbon atoms
- kDa:
-
Molecular weight unit (103 Da)
- M:
-
Molar concentration unit (mol.l−1)
- MPa:
-
Pressure unit (106 P=10 bars)
- OH group:
-
Hydroxyl group
- PE:
-
Protective effect
- PME:
-
Pectin methylesterase
- P50% :
-
Pressure of half deactivation for a settled treatment time (MPa)
- RML:
-
Rhizomucor miehei lipase
- t 1/2 :
-
Half-life (min)
References
Sztajer H, Maliszewska L, Wieczorck J (1998) Production of exogenous lipases by bacteria, fungi, and actinomycetes. Enzyme Microbial Technol 10:492–497
Ionita A, Moscovici M, Popa C, Vamanu A, Popa A, Dinu L (1997) Screening of yeast and fungal strains for lipolytic potential and determination of some biochemical properties of microbial lipases. J Mol Catal B Enzym 3:147–151
Klibanov AM (1989). Enzymatic catalysis in anhydrous organic solvents. Trends Biochem Sci 14:141–144
Malaca FX, Reyes HR, Garcia HS, Hill CG, Amundson CH (1992) Kinetics and mechanisms of reactions catalysed by immobilized lipases. Enzyme Microbial Technol 14:426–446
Pathel RN, Banerjee A, Nanduri V, Goswami A, Comezoglu FT (2003) Enzymatic resolution of racemic secondary alcohols by lipase B from Candida antartica. J Am Chem Soc 77:1015–1019
Jaeger KE, Eggert T (2002). Lipases for biotechnology. Curr Opin Biotechnol 13:390–397
West SI (1988) Enzymes in the food processing industry. Chem Brit 24(12):1220–1222
Miura T, Yamane T (1997) Screening for fungi that have lipolytic and acidolytic activity in biomass support particles. Biosci Biotechnol Biochem 61:1252–1257
Stocklëin W, Sztajer H, Menge U, Schmid RD (1993) Purification and properties of a lipase from Penicillium expansum. Biochim Biophys Acta 1168:181–189
Nagaoka K, Yamada Y (1973) Purification of mucor lipases and their properties. Agric Biol Chem 37(12):2791–2796
Ramarethinam S, Latha K, Rajalakshmi N (2002) Use of a fungal lipase for enhancement of aroma in black tea. Food Sci Technol Res 8(4):328–332
Knezevic Z, Bobic S, Milutinovic A, Obradovic B, Mojovic L, Burgarski B (2002) Alginate-immobilized lipase by electrostatic extrusion for the purpose of palm oil hydrolysis in lecithin/isooctane system. Process Biochem 38:313–318
Bornscheuer UT, Bessier C, Srinivas R, Krishna SH (2002) Optimizing lipases and related enzymes for efficient application. Trends Biotechnol 20:433–437
Polydera AC, Galanou E, Stoforos NG, Taoukis PS (2004) Inactivation kinetics of pectin methylesterase of greek Navel orange juice as a function of high hydrostatic pressure and temperature process conditions. J Food Eng 62:291–298
Noël-M.O M, Athés V, Combes D (2000) Combined effects of temperature and pressure on enzyme stability. High Pressure Res 19:317–322
Costa SA, Tzanov T, Carneiro AF, Paar A, Gübitz GM, Cavaco-Paulo A (2002) Studies stabilization of native catalase using additives. Enzyme Microbial Technol 30:387–391
Lozano P, Combes D, Iborra JL (1994) Effects of polyols on α-chymotrypsin thermostability a mechanistic analysis of the enzyme stabilization. J Biotechnol 35:9–18
Lullien-Pellerin V, Balny C (2002) High-pressure as a tool to study some proteins properties: conformational modification, activity and oligomeric dissociation. Innovative Food Sci Emerg Technol 3:209–221
Schubring R, Meyer C, Schlüter O, Boguslawski S, Knorr D (2003) Impact of high pressure assisted thawing on the quality of fillets from various fish species. Innovative Food Sci Emerg Technol 4:257–267
Riahi E, Ramaswamy HS (2003) High-pressure processing of apple juice: kinetics of pectin methyl esterase inactivation. Biotechnology 19:908–914
Chen H, Hoover G (2003) Pressure inactivation kinetics of Yersinia enterocolitica ATCC 35669. Int J Food Microbiol 87:161–171
Drews O, Weiss W, Reil G, Parlar H, Wait R, Görg A (2002) High pressure effects step-wise altered protein expression in Lactobacillus sanfranciscensis. Proteomics 2:765–774
Polydera AC, Stoforos NG, Taoukis PS (2003) Comparative shelf life study and vitamin C loss kinetics in pasteurised and high pressure processed reconstituted orange juice. J Food Eng 60:21–29
Hawley SA (1971) Reversible pressure–temperature denaturation of chymotrypsinogen. Biochemistry 10:2436–2442
Degraeve P, Rubens P, Lemay P, Heremans K (2002) In situ oservation of pressure-induced increased thermostability of two β-galactosidases with FT-IR spectroscopy in the diamond anvil cell. Enzyme Microbial Technol 31:673–684
Noël M, Combes D (2003) Rhizomucor miehei lipase: differential scanning calorimetry and pressure/temperature stability studies in presence of soluble additives. Enzyme Microbial Technol 33:299–308
Athès V, Lange R, Combes D (1998) Influence of polyols on the structural properties of Kluyveromyces lactis beta-galactosidase under high pressure. Eur J Biochem 255:206–12
Noël M, Combes D (2003) Effect of temperature and pressure on Rhizomucor miehei lipase stability. J Botechnol 102:23–32
Vannieuwenburgh C, Guibert A, Combes D (1998) A reliable method for precise initial rate determination of enzyme activity under high hydrostatic pressure with simple apparatus—application to Aspergillus niger fructosyl-transferase activity measurements. Biotechnol Tech 12:931–934
Longo MA, Combes D (1995). A novel chemoenzymatic glycosylation strategy: application to lysozyme modification. FEBS Lett 375:63–66
Huge-Jensen B, Galluzzo DR, Jensen RG (1987) Partial purification and characterisation of free and immobilized lipases from Mucor miehei. Lipids 22:559–565
Lozano P, Cano J, Iborra JL, Manjón A (1993) Influence of polyhydric cosolvent on papain thermostability. Enzyme Microbial Technol 15:868–873
Balny C, Mozhaev VV, Lange R (1997) Hydrostatic pressure and protein: Basic concepts and new data. Comp Biochem Physiol 116:299–304
Nawani N, Kaur J (2000) Purification, characterization and thermostability of lipase from a thermophilic Bacillus sp J33. Mol Cell Biochem 206:91–96
Zhang N, Suen WC, Windsor W, Xiao L, Madison V, Zaks A (2003) Improving tolerance of Candida antarctica lipase B towards irreversible thermal inactivation through directed evolution. Protein Eng 16:599–605
Combes D (1992) Biocatalysis in non conventional media effect of enzyme microenvironment. Prog Biotechnol 8:45–52
Acknowledgments
Marilyne Noël (pHD student) and authors are very grateful to Peter Winterton for improving the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noël, M., Lozano, P. & Combes, D. Polyhydric alcohol protective effect on Rhizomucor miehei lipase deactivation enhanced by pressure and temperature treatment. Bioprocess Biosyst Eng 27, 375–380 (2005). https://doi.org/10.1007/s00449-005-0417-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-005-0417-1