Abstract
Recently commenced host–parasite interactions provide an excellent opportunity to study co-evolutionary processes. Multi-host systems are especially informative because variation in virulence between hosts and temporal changes provides insight into evolutionary dynamics. However, empirical data under natural conditions are scarce. In the present study, we investigated the interaction between Darwin’s finches and the invasive fly Philornis downsi whose larvae feed on the blood of nestlings. Recently, however, the fly has changed its behavior and now also attacks incubating females. Two sympatric hosts are affected differently by the parasite and parasite load has changed over time. Our study observed a reversal of trends described two decades ago: while, currently, small tree finches (Camarhynchus parvulus) experience significantly higher parasite load than warbler finches (Certhidea olivacea), this was the opposite two decades ago. Currently, fledging success is higher in warbler finches compared to small tree finches. Our data indicate that not only intensity but also timing of infestation influences hosts’ reproductive success and parasite fitness. During incubation, prevalence was higher in warbler finches, but once chicks had hatched, prevalence was 100% in both species and parasite load was higher in small tree finches. Furthermore, our results suggest faster development and higher reproductive success of P. downsi in small tree finch nests. A change in host preference driven by larvae competition could have led to the reversal in parasite load.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites cause direct or indirect fitness loss by impairing reproduction and survival (Clayton and Moore 1997; Lehmann 1993; Loye and Zuk 1991; Price 1980; Tschirren et al. 2009) and, hence, they represent a major selective force. Virulence refers to the degree of fitness loss caused by pathogens and parasites. It is the result of complex interactions between host traits (resistance and tolerance) and parasite traits (exposure and pathogenicity; Johnson et al. 2012; Råberg 2014; Råberg et al. 2009). Thus, virulence can vary greatly between and within-host species (Bull 1994), which in turn influences if and how an evolutionarily stable equilibrium between hosts and parasites is reached (Rigaud et al. 2010). Multi-host systems are especially informative because variation in virulence between hosts and changes in virulence over time provide an excellent opportunity to observe the evolutionary dynamics of host–parasite interactions. However, empirical data gathered under natural conditions that show the interplay between tolerance, resistance, virulence and parasite productivity in multi-host systems are scarce (Manzoli et al. 2018). Recently established host–parasite interactions (e.g., due to an invasion by a new parasite species) allow the tracking of adaptative processes in hosts and parasites from the outset.
An example of one recently established, dynamically changing multi-host model field system is the interaction between the invasive ectoparasitic fly Philornis downsi and different species of Darwin’s finches, which are highly affected hosts of this fly on the Galápagos Islands. This obligate bird parasite was introduced to the Galápagos archipelago in the 1960s (Causton et al. 2006; Kleindorfer and Sulloway 2016). Free-living adult flies deposit their eggs in bird’s nests where their hematophagous larvae hatch (Fessl et al. 2006b). The first instar larvae usually develop in the chicks’ nostrils, whereas the second and third instar larvae live in the bottom layer of the nest and suck blood from the nestlings (Dudaniec and Kleindorfer 2006; Fessl et al. 2006b; O’Connor et al. 2010a), causing severe fitness loss in their avian hosts (reviewed in Fessl et al. 2017).
However, the level of virulence of P. downsi differs between host species on the Galápagos Islands. Galápagos mockingbirds (Mimus parvulus) tolerate the introduced parasite well, while sympatric medium ground finches (Geospiza fortis) suffer high brood loss due to parasitism (Knutie et al. 2016). Similarly, our recent study showed that in the same habitat, warbler finches (Certhidea olivacea) are less affected by P. downsi than closely related small tree finches (Camarhynchus parvulus) (Cimadom et al. 2019).
The aim of the present study was to investigate the causes of the differences in P. downsi virulence between the warbler finch and the small tree finch. More specifically, we wanted to investigate how parasite intensity in different phases of the hosts’ breeding cycle influences host reproductive success and parasite developmental success.
High parasite intensity is one source of elevated virulence (Stearns and Koella 2008) but it is also important to consider the number of parasites in relation to host body mass (Knutie et al. 2016). Cimadom et al. (2019) showed that in an average nest, the parasite burden increased with chick mass: a 50% higher burden was found in small tree finches (~ 13 g) than in warbler finches (~ 8 g). The timing of infestation within the breeding cycle may also influence virulence. There are strong indications of a recent change in parasite infestation behavior on Santa Cruz Island. Previously, larvae were only found in nests once chicks had hatched, but since 2012, we have observed P. downsi larvae of all larval stages in nests during the incubation stage when no chicks were present (Cimadom et al. 2016). At this stage, it is likely that the larvae are feeding on incubating females (Huber et al. 2010; Koop et al. 2013). For newly hatched chicks, a small difference in total parasite intensity might lead to significant differences in virulence (Arendt 1985; Kleindorfer and Dudaniec 2016). Furthermore, as P. downsi likely increases its blood consumption with increasing larval size and larval stage, not only the total amount of larvae but also the size and/or type of instar present when chicks hatch might have a significant effect on the virulence of P. downsi.
For the parasite’s reproductive success, the developmental stage of the larvae when the host resource availability terminates (either dead chicks or fledged hosts) is also a relevant factor as it determines its fitness. Parasite fitness depends on how fast the larvae develop (which depends on host quality) and on the mean duration of host availability (which depends on virulence). It has been suggested that the change in infestation behavior shown by P. downsi might be a result of intraspecific competition between the parasites: due to increasing parasite intensities in hosts over the last two decades, hosts die at an earlier age which reduces the time during which resources are available for developing P. downsi larvae (Kleindorfer and Dudaniec 2016; Kleindorfer et al. 2014). The time window of host resource availability determines not only parasite pupation success but also pupae size and ultimately adult fecundity (Honek 1993; Kleindorfer et al. 2014; Quiroga and Reboreda 2013; Spalding et al. 2002). In newly established multi-host systems, parasite developmental speed and, thus, host suitability might differ between host species, which could lead to the evolution of host preferences.
In the present study, we investigated the dynamics of a multi-host system under natural conditions by analyzing longitudinal data on virulence, infestation patterns and parasite developmental success in two sympatric host species, the warbler finch and small tree finch. Specifically, we addressed the following questions: Are there differences between the two hosts in the intensity and timing of infestation within the breeding cycle? Does this affect virulence and/or developmental success of the parasite? Are there changes in P. downsi intensity between and within hosts over time?
Methods
Study site and nest monitoring
The study was conducted at the “Los Gemelos” site in the humid highland of Santa Cruz Island, Galápagos (S 00°37′20″–45″ W 90°23′00″–15″, 500–600 m a.s.l.) between 2012 and 2017 (except for 2013). The study site is one of the last remnants (ca. 100 ha) of native Scalesia pedunculata forest on Santa Cruz (Mauchamp and Atkinson 2011).
We monitored the nests of warbler finches and small tree finches over five breeding seasons from 2012 to 2017 (January–March 2012, January–April 2014, January–May 2015, January–April 2016 and January–April 2017). Nest monitoring followed the procedure described in Cimadom et al. (2014). Once a nest was determined to be in the incubating or feeding stage, a pole-mounted endoscopic camera (Findoo 3.6, dnt Innovation, 26789 Leer, Germany) was used to assess the number of eggs or nestlings present and to determine whether the nestlings were alive. Monitoring intervals were adjusted to the status of the nest: three-day intervals were used to monitor for nests during incubation, two-day intervals for nests with chicks. Nests were monitored daily around the presumed fledging date of chicks.
After breeding failure or successful fledging, all monitored nests were collected in separate, sealed plastic bags and dismantled in the laboratory on the same day to count the number of P. downsi larvae, pupae and empty puparia. Larvae were categorized as either Small (< 0.5 mm), Medium (5–10 mm) or Big (> 10 mm). Parasite intensity for a nest was defined as the total number of P. downsi individuals in the nest at the termination of breeding activity. Since it has been found that the intensity per chick decreases with increasing brood size—an indication of a parasite dilution effect (Dudaniec et al. 2007), the number of chicks could also have been relevant for this study. However, here we did not evaluate parasite intensity per chick because (1) clutch size did not differ between the two species (warbler finch: 2.28 ± 0.62, mean ± SD, N = 113; small tree finch: 2.38 ± 0.76, N = 78; Mann–Whitney test: U = 4798.5, p = 0.25), (2) the maximum number of chicks was not known for all nests and (3) the number of chicks changed over the nestling period, as chicks often die sequentially. The age of chicks at the termination of breeding activity was recorded for each nest as described in Cimadom et al. (2014). Chicks of both species fledge at about 14 days of age (Cimadom et al. 2014). Because of the onset of the breeding seasons differed between years, we calculated a standardized start of incubation for each nest as (DIS − minDIS)/(maxDIS − minDIS), where DIS is the date of incubation start for a given nest, minDIS is the earliest recorded date of incubation start and maxDIS is the latest recorded date of incubation start in each year (values range from 0 to 1).
Size of eclosed flies
To test for differences in the size of eclosed P. downsi flies, we measured the head width (eye to eye) to the nearest 0.1 mm with a caliper. P. downsi flies emerged from 26 small tree finch nests and 31 warbler finch nests in 2012.
Statistical analysis
All statistical analyses were performed using the program R, version 3.5.1 (R Core Team 2018). For all models described below, analysis-of-variance tables (type-II) were calculated using the Anova procedure in the car package (Fox and Weisberg 2011).
Philornis downsi prevalence and intensity
We used separate general linear models (GLMs; binomial family and logit link function) to test for the effect of host species, standardized start of incubation and year on P. downsi prevalence (P. downsi present or absent in nest) in nests that failed during the incubation phase (N = 109) and in nests with chicks (N = 528).
We compared parasite prevalence between abandoned nests and nests that failed for other reasons during incubation using Chi2-tests for each host species separately, pooling the data from all five years.
We used a GLM (quasi-Poisson error structure) to test for the effect of host species and year on P. downsi intensity in infested nests that failed during the incubation phase (N = 70).
Effects of host species, age of chicks at nest failure or fledging (linear and quadratic), the interaction term host species * age of chicks, and year on parasite intensity (number of P. downsi individuals per nest) were evaluated using a GLM with a negative-binomial error structure (because of overdispersion). For this analysis, only nests with chicks were used (N = 513).
Nesting outcome and chick age
We used GLMs (quasi-Poisson error structure) to test for differences between the two Darwin’s finch species and years (as co-variable) in chick age at the end of nesting activity (failure or fledged, N = 513) and in chick age at death (failed nests only, N = 315).
In a previous study, we found a higher nest failure rate in the small tree finch than in the warbler finch (Cimadom et al. 2019). Here, we used the same data to see if the previously described breeding failure was related to chick age. We did this using a GLM (Poisson distribution) where the number of failed nests was a dependent variable and the age of chicks, host species and the interaction between host species * age of chicks as independent variables. The total number of nests per host species and the year was defined as an offset variable, to calculate probability values for each chick age, host species and year combination.
Developmental success of the parasite
Philornis downsi undergoes three larval stages before pupation (Fessl et al. 2006b): the first, second and third instar stages. Only third instar larvae can pupate, so upon occurrence of host death, it is only larvae that have reached the third instar stage which can pupate and subsequently emerge as flies (Kleindorfer et al. 2014; Knutie et al. 2016). Thus, the total number of third instar larvae and of pupae provides a good estimate of the parasite’s developmental success. Philornis downsi eggs take at least 4–7 days to develop into pupae (Kleindorfer et al. 2014). This means that the age that chicks reach in the nest (which may vary between host species) is linked to parasite developmental success.
Big larvae are always third instar larvae. Medium larvae include both second and third instar larvae and Small larvae are either first or second instars. In 2012, all larvae (all three size categories together) from each nest were kept in separate Petri dishes (diameter 90 mm) that were lined with a small, piece of moistened paper towel. Pupae from the nests were stored in gauze-covered plastic cups (for each nest separately). Larvae and pupae were kept at 27 °C and 65% relative humidity until eclosion or death. In total, out of 724 Big and 633 Medium larvae collected, 913 pupated. If we assume that all Big larvae pupated, we can conclude that 29.9% of the medium larvae were also third instars that managed to pupate. We conservatively defined mature P. downsi larvae, which are no longer dependent on a living host, as the number of Big larvae found per nest. As a proxy for parasite developmental success, we used the total number of mature P. downsi larvae and pupae per nest. To test if the number of mature larvae and pupae differed between the two host species, we ran a GLM (quasi-Poisson error structure) with the total number of mature P. downsi larvae and pupae as the dependent variable, host species as the independent variable and year as a co-variable (N = 513).
The proportion of mature larvae and pupae per nest was used as an indicator of pupation success (following Kleindorfer et al. 2014). A detailed analysis of the proportion of mature larvae, evaluating the effects of host species, age of chicks at failure or fledging (linear and quadratic), year and the interaction of host species * age of chicks on the proportion of mature larvae and pupae within a nest was conducted by constructing a GLM with a quasi-binomial error structure and logit link function (N = 513).
In a further step, we focused on nests with young chicks (≤ 5 days old, N = 160), since for young chicks, a small difference in total parasite intensity might lead to significant differences in virulence (Arendt 1985; Kleindorfer and Dudaniec 2016). We tested to see whether there were differences between the host species in total parasite intensity (GLM with quasi-Poisson error structure), pupation success (GLM with quasi-binomial error structure) and the number of mature (big larvae and pupae) and immature (small and medium) larvae separately (GLMs with quasi-Poisson error structure). In all these models, the year was included as a co-variable.
To test whether the size of eclosed flies differed between the host species, we ran a linear mixed model (LMM) with fly eye distance as the dependent variable and host species, age of chicks at failure or fledging and sex of fly as fixed factors and nest provenance as a random factor (random intercept) using the package lme4 (Bates et al. 2015). The sex ratio of eclosed flies did not differ between the two host species and did not change over the breeding season (Causton et al. 2019) and was, therefore, not included in the analysis.
Long-term shifts in parasite intensity
To test for changes in P. downsi intensity over the last decade, we compared pooled data from 1998 to 2005 (Dudaniec et al. 2007) with our present data from 2012 to 2017 for the warbler finch and the small tree finch separately using one-sampled t-Tests.
Results
Philornis downsi prevalence and intensity during the incubation phase
Philornis downsi prevalence in nests that failed during incubation was significantly higher in warbler finch nests than in small tree finch nests (X2 = 16.62, df = 1, p ≪ 0.001, Fig. 1a, Table S1) and differed among years (X2 = 9.47, df = 4, p = 0.05, Fig. 1a, Table S1). Parasite prevalence in small tree finch nests that were being incubated ranged from 0 to 25% and from 14.3 to 80% in warbler finch nests being incubated—it was highest in 2012 in both species. Parasite prevalence in nests being incubated did not change within the breeding season (Χ2 = 0.08, df = 1, p = 0.77, Table S1).
Philornis downsi infestation in the incubation (left column) and nestling phase (right column). Difference in P. downsi prevalence (percentage of infested nests) in a incubating nests and b nests with chicks of the small tree finch (gray) and the warbler finch (white) from 2012 to 2017. c P. downsi intensity in infested small tree finch (gray) and warbler finch (white) nests in the incubation phase from 2012 to 2017. Boundaries of the boxes represent 1st and 3rd quartiles, the black line within the boxes marks the median and whiskers extend from the median to the largest and lowest value within 1.5*IQR (interquartile range). d Predicted P. downsi intensity (± 95% CI) in warbler finch nests (solid line) and small tree finch nests (dashed line) in the nestling phase in relation to chick age. Fitted lines indicate the model’s predicted values for each set of observed values of the independent variables. Raw data are plotted as dots (small tree finch in gray and warbler finch in white)
There was no significant difference in P. downsi intensity in infested incubation phase nests between the two host species (X2 = 0.004, df = 1, p = 0.95, Fig. 1c, Table S2) nor did it differ among study years (X2 = 4.43, df = 4, p = 0.35, Fig. 1c, Table S2). Median P. downsi intensity in both infested incubation phase warbler finch and small tree finch nests was 2.5 (warbler finch: range = 1-41, N = 52; small tree finch: range = 1-35, N = 18).
Prevalence and Philornis downsi intensity during the nestling phase
Over all five study years, P. downsi prevalence during the nestling phase did not differ between the two host species (X2 = 0.03, df = 1, p = 0.85, Fig. 1b, Table S1) but varied among years (X2 = 27.86, df = 4, p ≪ 0.001, Fig. 1b, Table S1). Prevalence in small tree finch nests ranged from 83% to 100% and from 85% to 100% in warbler finch nests and it was lowest in 2017 in both species (Table S3).
Analysis of P. downsi intensity per nest revealed that the parasite load per nest differed significantly between species (X2 = 55.1, df = 1, p ≪ 0.001, Fig. 1d, Table S2) with warbler finch nests containing fewer parasites (22.8 ± 1.0 parasites per nest, N = 290) than small tree finch nests (mean ± SE parasites per nest: 34.4 ± 1.6, N = 223). Furthermore, the parasite load of both species increased with chick age until Day 11 and then leveled off from Days 11 to 15 (linear X2 = 23.4, df = 1, p ≪ 0.001; and quadratic X2 = 8.9, df = 1, p = 0.003, Fig. 1d, Table S2). This relationship did not differ significantly between the two host species (host species*chick age: X2 = 0.14, df = 1, p = 0.70, Table S2). The parasite load differed among years (X2 = 13.0, df = 4, p = 0.011, Table S2) with 2017 nests exhibiting the lowest numbers of P. downsi (Table S3).
Nesting outcome and chick age
The age of warbler finch chicks at the end of nest activity (failure or fledging) was significantly greater than that of small tree finch chicks (X2 = 57.8, df = 1, p ≪ 0.001, Table S3 and S4) and differed among years (X2 = 21.0, df = 4, p = 0.001, Table S4). In both species, chick age was greatest in 2017 (mean ± SE age of warbler finches: 12.5 ± 0.46 Days; mean ± SE age of small tree finches: 8.8 ± 0.80 Days, Table S3). When data were pooled for all five study years, mean chick age was 10.7 ± 0.28 (mean ± SE) in the warbler finch and 7.4 ± 0.29 in the small tree finch. However, mean age at chick death did not differ between species (X2 = 0.62, df = 1, p = 0.43, Table S4) or among years (X2 = 3.37, df = 4, p = 0.50, Table S4). When data were pooled for all five study years, age at chick death was 6.3 ± 0.3 (mean ± SE) in the warbler finch and 6.0 ± 0.3 in the small tree finch (Table S3).
The probability of failure was lower for warbler finch nests compared to small tree finch nests (X2 = 60.7, p < 0.001, Fig. 2, Table S5) and decreased in both finch species with increasing age of the chicks (X2 = 29.9, p < 0.001, Fig. 2, Table S5). This relationship did not differ between the two species (host species*chick age: X2 = 0.65, p = 0.42, Table S5).
Predicted probability of failure (± 95% CI) of warbler finch (solid line) and small tree finch nests (dashed line) in relation to chick age. Fitted lines indicate the model’s predicted values for each set of observed values of the independent variables. Raw data are plotted as dots (small tree finch in gray and warbler finch in white)
Developmental success of the parasite
The number of mature larvae and pupae in nests with chicks was significantly higher in the small tree finch than the warbler finch (X2 = 8.70, df = 1, p = 0.003, Table S6) and did not differ significantly among years (X2 = 1.31, df = 4, p = 0.86, Table S6). Over all five study years, the mean number of mature larvae and pupae was 20.6 ± 1.4 (mean ± SE) per nest in small tree finch nests and 15.8 ± 0.8 per nest in warbler finch nests.
The proportion of mature larvae and pupae increased significantly with chick age (linear: X2 = 48.28, df = 1, p ≪ 0.001; quadratic: X2 = 1.78, df = 1, p = 0.18, Table S6). This increase, however, was different in the two Darwin’s finch species (host species*chick age: X2 = 21.94, df = 1, p ≪ 0.001, Table S6). In warbler finch nests, the proportion of mature P. downsi larvae and pupae was higher for nests with young chicks but increased more slowly and reached lower levels in nests with older chicks compared to small tree finch nests (Fig. 3). Furthermore, the proportion of mature larvae and pupae differed among years (X2 = 43.20, df = 4, p ≪ 0.001, Table S6): it was lowest in 2012 and 2016 and highest in 2014 and 2017 (Table S3).
Predicted relative frequency of mature P. downsi larvae (± 95% CI) in nests of the warbler finch (solid line) and the small tree finch (dashed line) in relation to the age of host chicks. Fitted lines indicate the model’s predicted values for each set of observed values of the independent variables. Raw data are plotted as dots (small tree finch in gray and warbler finch in white)
Analysis of the early nestling phase (inclusion only of nests with ≤ 5-day-old chicks) confirmed lower parasite intensity (X2 = 23.47, df = 1, p ≪ 0.001, Table S7) and a higher proportion of mature larvae and pupae (X2 = 11.53, df = 1, p = 0.001, Table S7) in warbler finch nests compared to small tree finch nests. The number of mature larvae and pupae in nests with ≤ 5-day-old chicks did not differ between the two host species (X2 = 0.18, df = 1, p = 0.67, Table S7), but small tree finch nests with young chicks contained more immature (Small and Medium) larvae than warbler finch nests (X2 = 32.23, df = 1, p ≪ 0.001, Table S7).
Mean size of eclosed P. downsi flies did not differ between host species (mean ± SE eye distance for warbler finch nests was 2.72 ± 0.20 mm and 2.55 ± 0.23 mm for small tree finch nests; X2 = 0.04, df = 1, p = 0.84, Table S8). However, size increased with chick age (X2 = 61.08, df = 1, p ≪ 0.01, Fig. 4, Table S8) and male flies were significantly bigger than female flies (X2 = 40.31, df = 1, p ≪ 0.001, Fig. 4, Table S8).
Predicted eye distance (± 95% CI) of female (solid line) and male (dashed line) P. downsi flies in relation to the age of host chicks. Fitted lines indicate the model’s predicted values for each set of observed values of the independent variables. Raw data are plotted as dots (small tree finch in gray and warbler finch in white)
Long-term shifts in parasite intensity
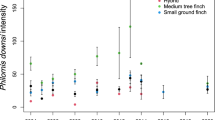
Compared to pooled data from 1998 to 2005 (Dudaniec et al. 2007), P. downsi intensity in nests with chicks ≥ 6 days has decreased in the warbler finch (one-sample t Test: N1998-2005 = 21, N2012-2017 = 228, t = 3.4, p = 0.002, Fig. 3) and increased in the small tree finch (one-sample t-Test: N1998–2005 = 33, N2012–2017 = 125, t = 2.6, p = 0.011, Fig. 5).
Number of P. downsi (mean ± SE) in warbler finch and small tree finch nests with nestlings ≥ 6 days old in the years 1998–2005 (Dudaniec et al. 2007) and 2012–2017
Discussion
Our study showed that infestation patterns, as manifested by parasite prevalence and intensity, differed between the warbler finch and the small tree finch. Furthermore, these patterns changed within the breeding cycle suggesting a difference in the timing of infestation between the two hosts. During the incubation phase, P. downsi was more prevalent in the warbler finch than in the small tree finch and parasite intensity did not differ in infested incubation phase nests between the two hosts. However, once chicks hatched, prevalence was over 80% in both species, but small tree finch nests had higher parasite numbers than warbler finch nests over the whole nestling phase.
Differences in parasite intensity between the two hosts could be caused by differences in host attractiveness and/or host suitability. Currently, it is not clear how female flies find their hosts but olfactory cues are likely to be involved (Cha et al. 2016). Since small tree finch chicks are bigger than warbler finch chicks, they probably produce more olfactory cues which may make it easier for flies to detect them. Host suitability, on the other hand, may depend on factors such as nest characteristics (Kleindorfer and Dudaniec 2009; Sage et al. 2018) and host body mass (Kleindorfer and Dudaniec 2009). The fact that small tree finches have larger nests and larger chicks could explain why parasite intensity is higher than in warbler finch nests once chicks have hatched. However, this hypothesis is not in line with the finding of greater parasite intensity in warbler finches compared to small tree finches between 1998 and 2005.
One explanation for higher parasite prevalence in warbler finch nests during incubation could be that in some years, the breeding onset of warbler finches is earlier than of small tree finches. In these years, their incubation phase nests are the first or only available food source for the parasite at the beginning of the breeding season. However, parasite prevalence in nests being incubated did not change within the breeding season (standardized start of incubation) in either of the two hosts. Alternatively, the pattern could be a consequence of the overall higher density of warbler finch nests. Warbler finches are approximately 1.6 times more common than small tree finches (Cimadom et al. 2014, unpublished data) and it is possible that flies using only visual cues will encounter them more easily. The cause of higher prevalence of P. downsi in warbler finch nests during the incubation phase remains unclear. Detailed studies on fly behavior with cameras at the nest could provide relevant data such as when and how often adult flies visit the nests during the incubation and nestlings’ phase. Additionally, genetic studies like those presented in Dudaniec et al. (2010) could reveal the number of different female flies and the number of eggs they oviposited in each nest over the entire host’s breeding cycle.
Implications for parasite virulence
Virulence may depend not only on the parasite load relative to chick mass and mechanisms of tolerance (e.g., Knutie et al. 2016) but also on the age at which the hosts are exposed to the parasite. The magnitude of the difference in virulence between the two host species is higher in young chicks. In nests with young chicks, the abundance of mature P. downsi larvae is the same in both species but small tree finch nests contain more immature larvae. This latter difference is likely to be the cause of the increased virulence in young small tree finch chicks. More specifically, first instar larvae develop predominantly in the nares (or ear cavities) of chicks (Fessl et al. 2006b), where they cause tissue damage and beak malformation (Galligan and Kleindorfer 2009), which could contribute to the negative fitness effects of the parasite during this larval stage.
Implications for parasite outcome
As expected, the proportion of mature larvae and pupae increased with chick age in both host species. We were able to confirm the findings of Kleindorfer et al. (2014) that increasing duration of chick survival corresponded to a higher pupation success of P. downsi larvae. In addition, there was a positive relationship between fly size and chick age, indicating the potentially higher reproductive success of flies that had more time to develop. However, the relationship between the proportion of mature larvae and pupae and chick age differed in the two hosts. In the warbler finch, the proportion of mature larvae and pupae was higher early in the nestling phase and increased more slowly; whereas in the small tree finch, it increased faster and reached higher levels in older nests. Kleindorfer et al. (2014) showed that P. downsi pupation success differed between the small ground finch and the small and medium tree finch but the factors causing the difference were unknown. In the closely related Philornis torquans, parasite reproductive success differed greatly between three host species (Manzoli et al. 2018) and depended on a complex interplay between parasite intensity, tolerance and resistance. In the present study, the higher proportion of mature larvae and pupae in warbler finch nests directly after hatching could be due to the higher infestation of incubating warbler finch nests compared to small tree finch nests. The finding that parasite intensity increases with chick age in both species, but that the proportion of mature larvae and pupae increased faster in the small tree finch than in the warbler finch might indicate that P. downsi larvae develop faster when parasitizing small tree finch chicks than warbler finch chicks. There are different possible (non-mutually exclusive) explanations for the differential increase in the proportion of mature larvae and pupae between the two hosts: (1) differences in the host-associated larval gut microbiome (Ben-Yosef et al. 2017), (2) differences in resistance due to differing immunocompetence, for example, the presence of Philornis-binding antibodies (Huber et al. 2010), and (3) difference in host blood quality due to differences in host diet. The warbler finch is insectivorous while the small tree finch is omnivorous (Filek et al. 2018) and thus, it is likely that their blood also differs in the ratio between carbohydrate versus nitrogen (the C:N ratio) ratio which is an indicator for food quality (reviewed in Aalto et al. 2015). These hypotheses could be tested by studying host and parasite microbiomes and measuring immunocompetence and blood quality (e.g., the C:N ratio) in relation to P. downsi parasitism. In addition to a more rapid increase in the proportion of mature larvae and pupae, small tree finch nests also yield a higher abundance of mature larvae and pupae at host resource termination because of the overall higher parasite intensity in these nests. Based on this body of evidence, it seems that the small tree finch is currently the better host for P. downsi.
Change over time
We have witnessed a dynamic change in the relationship between the parasite and the two hosts over the last two decades: while parasite intensity increased in the small tree finch, it decreased in the warbler finch. One possible explanation for this pattern is the host specificity of P. downsi strains. Such host specificity is assumed to be based on genetic differences or differential patterns of expression (phenotypic acclimation) between parasite strains (reviewed in Little et al. 2006). If parasite fitness differs between P. downsi strains, parasite intensity could decrease or increase independently between hosts via differential survival of host-specific parasite strains. Under this scenario, each host-specific strain would be dependent on its host, which could potentially lead to stable host–parasite relationships between each host–parasite dyad (Papkou et al. 2016). However, genetic studies conducted to date indicate that P. downsi is a generalist, showing low genetic differentiation within the population in the Galápagos and that multiple mating and multiple nest infestations are common in this species (Dudaniec et al. 2008; Dudaniec et al. 2010). But it should be noted that these studies were conducted over ten years ago and did not focus on genetic variation between P. downsi attacking different hosts.
Another possible explanation for changes in parasite intensity over time are changes in host preference. The developmental time window to reach pupation is short and may have decreased over the last two decades (Kleindorfer et al. 2014). Currently, nestling failure is high and the average age of chick death is around 6 days in both hosts—the minimum developmental time window from egg-laying to pupation in P. downsi. Under such conditions, a parasite should prefer hosts that are conducive to fast development. In this scenario, host preference driven by competition between parasites for host resources would lead to the dynamic changes we currently observe. Multi-host systems do not necessarily lead to a stable equilibrium between the parasite and each host species. In the presence of reservoir hosts of differing vulnerability, parasite populations can continue to be at high levels in a robust host even if other, more vulnerable hosts are decreasing (reviewed in de Castro and Bolker 2005; Woolhouse et al. 2001). This could even lead to extinction of some host species (Fessl et al. 2017; Lafferty and Gerber 2002). The different outcomes of these two scenarios have differing implications with respect to the conservation of the unique land bird community of the Galápagos Islands. Hence it is crucial to understand the mechanisms and selective forces underlying this dynamic multi-host–parasite system, so that the most effective management strategies may be implemented. Host-specific parasite strains would call for specific management strategies for each host–parasite dyad, especially for rare species close to extinction like the mangrove finch (Fessl et al. 2010) or the medium tree finch (Dvorak et al. 2017; O’Connor et al. 2010b). In the second scenario, which does not predict a stable host–parasite equilibrium, P. downsi management should be twofold: first, the entire parasite population must be reduced and second, specific protection measures must be established for rare host species. Several studies have shown that Darwin’s finch chicks can be protected effectively by treating nests with the insecticide permethrin, which substantially reduces P. downsi numbers (Causton et al. 2019; Fessl et al. 2006a; Knutie et al. 2014; Knutie et al. 2016; Koop et al. 2013; Koop et al. 2011; O’Connor et al. 2014).
In summary, the present study provides new insights into the dynamics of the host–parasite interaction between P. downsi and two species of Darwin’s finches that may be useful in future efforts to design targeted P. downsi control measures in the Galápagos. We found differences in parasite intensity and prevalence between the two hosts and within the breeding cycle. During incubation, the parasitic fly infested warbler finch nests more frequently but once chicks hatched small tree finch nests contained more larvae, which could explain the higher virulence in this host. Additionally, long-term data indicate that infestation patterns of the small tree finch and the warbler finch have reversed during the last decade and results suggest faster larval development in small tree finch nests potentially leading to P. downsi preference for the better host.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aalto SL, Decaestecker E, Pulkkinen K (2015) A three-way perspective of stoichiometric changes on host-parasite interactions. Trends Parasitol 31:333–340. https://doi.org/10.1016/j.pt.2015.04.005
Arendt WJ (1985) PHILORNIS Ectoparasitism Of Pearly-Eyed Thrashers. 1. Impact on growth and development of nestlings. Auk 102:270–280. https://doi.org/10.2307/4086769
Bates D, Machler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Ben-Yosef M et al (2017) Host-specific associations affect the microbiome of Philornis downsi, an introduced parasite to the Galapagos Islands. Mol Ecol 26:4644–4656. https://doi.org/10.1111/mec.14219
Bull JJ (1994) Perspective—virulence. Evolution 48:1423–1437. https://doi.org/10.2307/2410237
Causton CE, Peck SB, Sinclair BJ, Roque-Albelo L, Hodgson CJ, Landry B (2006) Alien insects: threats and implications for conservation of Galapagos Islands. Ann Entomol Soc Am 99:121–143
Causton CE et al (2019) Population dynamics of an invasive bird parasite, Philornis downsi (Diptera: Muscidae), in the Galapagos Islands. PLoS ONE. https://doi.org/10.1371/journal.pone.0224125
Cha DH et al (2016) Identification and optimization of microbial attractants for philornis downsi, an invasive fly parasitic on galapagos birds. J Chem Ecol 42:1101–1111. https://doi.org/10.1007/s10886-016-0780-1
Cimadom A et al (2014) Invasive parasites, habitat change and heavy rainfall reduce breeding success in Darwin’s finches. PLoS ONE 9:10. https://doi.org/10.1371/journal.pone.0107518
Cimadom A et al (2016) Darwin’s finches treat their feathers with a natural repellent. Sci Rep 6:9. https://doi.org/10.1038/srep34559
Cimadom A, Jäger H, Schulze CH, Hood-Nowotny R, Wappl C, Tebbich S (2019) Weed management increases the detrimental effect of an invasive parasite on arboreal Darwin’s finches. Biol Conserv 233:93–101. https://doi.org/10.1016/j.biocon.2019.02.025
Clayton DH, Moore J (1997) Host-parasite evolution: general principles and avian models. Oxford University Press, Oxford
de Castro F, Bolker B (2005) Mechanisms of disease-induced extinction. Ecol Lett 8:117–126. https://doi.org/10.1111/j.1461-0248.2004.00693.x
Dudaniec RY, Kleindorfer S (2006) Effects of the parasitic flies of the genus Philornis (Diptera: Muscidae) on birds. Emu 106:13–20
Dudaniec RY, Fessl B, Kleindorfer S (2007) Interannual and interspecific variation in intensity of the parasitic fly, Philornis downsi, in Darwin’s finches. Biol Conserv 139:325–332. https://doi.org/10.1016/j.biocon.2007.07.006
Dudaniec RY, Gardner MG, Donnellan S, Kleindorfer S (2008) Genetic variation in the invasive avian parasite, Philornis downsi (Diptera, Muscidae) on the Galápagos archipelago. BMC Ecol 8:13. https://doi.org/10.1186/1472-6785-8-13
Dudaniec RY, Gardner MG, Kleindorfer S (2010) Offspring genetic structure reveals mating and nest infestation behaviour of an invasive parasitic fly (Philornis downsi) of Galapagos birds. Biol Invasions 12:581–592. https://doi.org/10.1007/s10530-009-9464-x
Dvorak M et al (2017) Conservation status of landbirds on Floreana: the smallest inhabited Galapagos Island. J Field Ornithol 88:132–145. https://doi.org/10.1111/jofo.12197
Fessl B, Kleindorfer S, Tebbich S (2006a) An experimental study on the effects of an introduced parasite in Darwin’s finches. Biol Conserv 127:55–61. https://doi.org/10.1016/j.biocon.2005.07.013
Fessl B, Sinclair BJ, Kleindorfer S (2006b) The life-cycle of Philornis downsi (Diptera: Muscidae) parasitizing Darwin’s finches and its impacts on nestling survival. Parasitology 133:739–747. https://doi.org/10.1017/s0031182006001089
Fessl B et al (2010) How to save the rarest Darwin’s finch from extinction: the mangrove finch on Isabela Island. Philos Trans R Soc Lond B Biol Sci 365:1019–1030. https://doi.org/10.1098/rstb.2009.0288
Fessl B, Heimpel GE, Causton CE (2017) Invasion of an avian nest parasite, Philonris downsi, to the Galapagos Islands: colonization history, adaptations to novel ecosystems, and conservation challenges. In: Parker PG (ed) Disease ecology: galapagos birds and their parasites. Springer, Dordrecht
Filek N, Cimadom A, Schulze CH, Jäger H, Tebbich S (2018) The impact of invasive plant management on the foraging ecology of the Warbler Finch (Certhidea olivacea) and the Small Tree Finch (Camarhynchus parvulus) on Galapagos. J Ornithol 159:129–140. https://doi.org/10.1007/s10336-017-1481-4
Fox J, Weisberg S (2011) An R Companion to applied regression, 2nd edn. Sage Publications, London
Galligan TH, Kleindorfer S (2009) Naris and beak malformation caused by the parasitic fly, Philornis downsi (Diptera: Muscidae), in Darwin’s small ground finch, Geospiza fuliginosa (Passeriformes: Emberizidae). Biol J Linn Soc 98:577–585. https://doi.org/10.1111/j.1095-8312.2009.01309.x
Honek A (1993) Intraspecific variation in body size and fecundity in insects—a general relationship. Oikos 66:483–492. https://doi.org/10.2307/3544943
Huber SK et al (2010) Ecoimmunity in Darwin’s finches: invasive parasites trigger acquired immunity in the medium ground finch (Geospiza fortis). PLoS ONE 5:e8605. https://doi.org/10.1371/journal.pone.0008605
Johnson PTJ, Rohr JR, Hoverman JT, Kellermanns E, Bowerman J, Lunde KB (2012) Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecol Lett 15:235–242. https://doi.org/10.1111/j.1461-0248.2011.01730.x
Kleindorfer S, Dudaniec RY (2009) Love thy neighbour? Social nesting pattern, host mass and nest size affect ectoparasite intensity in Darwin’s tree finches. Behav Ecol Sociobiol 63:731–739. https://doi.org/10.1007/s00265-008-0706-1
Kleindorfer S, Dudaniec RY (2016) Host-parasite ecology, behavior and genetics: a review of the introduced fly parasite Philornis downsi and its Darwin’s finch hosts. BMC Zool 1:1. https://doi.org/10.1186/s40850-016-0003-9
Kleindorfer S, Sulloway FJ (2016) Naris deformation in Darwin’s finches: Experimental and historical evidence for a post-1960s arrival of the parasite Philornis downsi. Global Ecol Conserv 7:122–131. https://doi.org/10.1016/j.gecco.2016.05.006
Kleindorfer S, Peters KJ, Custance G, Dudaniec RY, O’Connor JA (2014) Changes in Philornis infestation behavior threaten Darwin’s finch survival. Curr Zool 60:542–550. https://doi.org/10.1093/czoolo/60.4.542
Knutie SA, McNew SM, Bartlow AW, Vargas DA, Clayton DH (2014) Darwin’s finches combat introduced nest parasites with fumigated cotton. Curr Biol 24:R355–R356
Knutie SA et al (2016) Galapagos mockingbirds tolerate introduced parasites that affect Darwin’s finches. Ecology 97:940–950. https://doi.org/10.1890/15-0119.1
Koop JAH, Huber SK, Laverty SM, Clayton DH (2011) Experimental demonstration of the fitness consequences of an introduced parasite of Darwin’s finches. PLoS ONE. https://doi.org/10.1371/journal.pone.0019706
Koop JA, Owen JP, Knutie SA, Aguilar MA, Clayton DH (2013) Experimental demonstration of a parasite-induced immune response in wild birds: Darwin’s finches and introduced nest flies. Ecol Evol 3:2514–2523. https://doi.org/10.1002/ece3.651
Lafferty KD, Gerber LR (2002) Good medicine for conservation biology: The intersection of epidemiology and conservation theory. Conserv Biol 16:593–604. https://doi.org/10.1046/j.1523-1739.2002.00446.x
Lehmann T (1993) Ectoparasites - direct impact on host fitness. Parasitol Today 9:8–13. https://doi.org/10.1016/0169-4758(93)90153-7
Little TJ, Watt K, Ebert D (2006) Parasite-host specificity: experimental studies on the basis of parasite adaptation. Evolution 60:31–38
Loye JE, Zuk M (1991) Bird-parasite interaction: ecology. Oxford University Press, Oxford, Evolution and Behaviour
Manzoli DE et al (2018) Contrasting consequences of different defence strategies in a natural multihost-parasite system. Int J Parasitol 48:445–455. https://doi.org/10.1016/j.ijpara.2017.11.001
Mauchamp A, Atkinson R (2011) Rapid, recent and irreversible habitat loss: scalesia forest on the Galapagos Islands. Galapagos Rep 2009–2010:108–112
O’Connor JA, Robertson J, Kleindorfer S (2010a) Video analysis of host–parasite interactions in nests of Darwin’s finches. Oryx 44:588–594. https://doi.org/10.1017/s0030605310000086
O’Connor JA, Sulloway FJ, Kleindorfer S (2010b) Avian population survey in the Floreana highlands: is Darwin’s Medium Tree Finch declining in remnant patches of Scalesia forest? Bird Conserv Int 20:343–353. https://doi.org/10.1017/s0959270910000195
O’Connor JA, Robertson J, Kleindorfer S, Herberstein M (2014) Darwin’s finch begging intensity does not honestly signal need in parasitised nests. Ethology 120:228–237. https://doi.org/10.1111/eth.12196
Papkou A, Gokhale CS, Traulsen A, Schulenburg H (2016) Host-parasite coevolution: why changing population size matters. Zoology 119:330–338. https://doi.org/10.1016/j.zool.2016.02.001
Price PW (1980) Evolutionary biology of parasites. Princeton University Press, Princeton
Quiroga MA, Reboreda JC (2013) Sexual differences in life history traits of Philornis seguyi (Diptera: Muscidae) parasitizing house wrens (Troglodytes aedon). Ann Entomol Soc Am 106:222–227. https://doi.org/10.1603/an12084
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Råberg L (2014) How to live with the enemy: understanding tolerance to parasites. PLoS Biol. https://doi.org/10.1371/journal.pbio.1001989
Råberg L, Graham AL, Read AF (2009) Decomposing health: tolerance and resistance to parasites in animals. Philos Trans R Soc B 364:37–49. https://doi.org/10.1098/rstb.2008.0184
Rigaud T, Perrot-Minnot MJ, Brown MJF (2010) Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc R Soc B 277:3693–3702. https://doi.org/10.1098/rspb.2010.1163
Sage R, Boulton RA, Lahuatte PF, Causton CE, Cloutier R, Heimpel GE (2018) Environmentally cued hatching in the bird-parasitic nest fly Philornis downsi. Entomol Exp Et Appl 166:752–760. https://doi.org/10.1111/eea.12721
Spalding MG, Mertins JW, Walsh PB, Morin KC, Dunmore DE, Forrester DJ (2002) Burrowing fly larvae (Philornis porteri) associated with mortality of eastern bluebirds in Florida. J Wildlife Dis 38:776–783. https://doi.org/10.7589/0090-3558-38.4.776
Stearns SC, Koella JC (2008) Evolution in health and disease. Oxford University Press, Oxford
Tschirren B, Siitari H, Saladin V, Richner H (2009) Transgenerational immunity in a bird-ectoparasite system: do maternally transferred antibodies affect parasite fecundity or the offspring’s susceptibility to fleas? Ibis 151:160–170. https://doi.org/10.1111/j.1474-919X.2008.00880.x
Woolhouse MEJ, Taylor LH, Haydon DT (2001) Population biology of multihost pathogens. Science 292:1109–1112. https://doi.org/10.1126/science.1059026
Acknowledgments
We thank David Anchundia, Luke Bonifacio, Nikolaus Filek, Nina Gallmetzer, Fidelino Gaona, Patricio Herrera, Nicholas Heimpel, Eileen Heyer, Wilson Iñiguez, Sophia Stankewitz, Denis Mosquera, Xavier Pilataxi, Ingrid Rabitsch Musso, Paul Schmidt Yáñez, Angel Ulloa, Christian Wappl and Julia Zarfl for field assistance. We are grateful to Caroline Raby and Irmgard Teschke for improving the English and Sonia Kleindorfer and Heinz Richner for comments on the manuscript. We thank the Charles Darwin Research Station for logistical support and the Galápagos National Park Directorate for their help. Permission for this study was granted by the Galápagos National Park Directorate (yearly Permits: PC-54-11, PC-02-14, PC-04-15, MAE-DPNG/DGA-2016-0012, PC-02-17).
Funding
Open access funding provided by Austrian Science Fund (FWF). The study was funded by the FWF (project number P 26556-B22 granted to Sabine Tebbich), the Ethologische Gesellschaft e.V., a focus of excellence grant to Sabine Tebbich and scholarships from the University of Vienna.
Author information
Authors and Affiliations
Contributions
AC and ST conceived and designed the study. AC and ST conducted fieldwork. AC analyzed the data. AC and ST wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Indrikis Krams.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cimadom, A., Tebbich, S. Timing of infestation influences virulence and parasite success in a dynamic multi-host–parasite interaction between the invasive parasite, Philornis downsi, and Darwin’s finches. Oecologia 195, 249–259 (2021). https://doi.org/10.1007/s00442-020-04807-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04807-5