Abstract
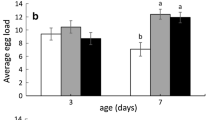
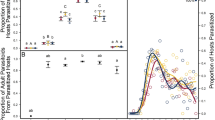
Parasitoid lifespan is influenced by nutrient availability, thus the lifespan of parasitoids that rely on their hosts for nutritional resources (either via host feeding or by consuming honeydew) should vary with host density. We assessed the survival and reproduction of one such species, Aphelinus certus—a parasitoid of the soybean aphid, Aphis glycines—over a range of host densities using a laboratory assay. We found a positive, asymptotic relationship between host density and the lifespan and fecundity of A. certus that was supported by a traditional survivorship analysis as well as a logistic model. Parasitoids from this assay were also used to develop a wing wear index relating setae damage to parasitoid age. This index was used to estimate the life expectancy of field-collected parasitoids, which was shorter than the life expectancy of laboratory-reared female parasitoids. Finally, host-density-dependent parasitoid lifespan was incorporated into a coupled-equations matrix population model that revealed that decreasing the degree of host density dependence leads to higher equilibrium host densities and changes in the quality of equilibrium (e.g. stable limit cycles). These results detail the relatively unstudied phenomenon of host-density-dependent parasitoid lifespan and suggest that differences between laboratory- and field-determined parasitoid life expectancy have important implications for population dynamics and the biological control of insects.








Similar content being viewed by others
Data availability
Data are available through the Data Repository for the University of Minnesota (DRUM) at https://doi.org/10.13020/rhbq-rc90.
References
Bai B, MacKauer M (1990) Oviposition and host-feeding patterns in Aphelinus asychis (Hymenoptera: Aphelinidae) at different aphid densities. Ecol Entomol 15:9–16
Briggs CJ, Nisbet RM, Murdoch WW, Collier TR, Metz JAJ (1995) Dynamical effects of host-feeding in parasitoids. J Anim Ecol 64:403–416
Casas J, Pincebourde S, Mandon N, Vannier F, Poujol R, Giron D (2005) Lifetime nutrient dynamics reveal simultaneous capital and income breeding in a parasitoid. Ecology 86:545–554
Cate RH, Sauer JR, Eikenbary ED (1974) Demonstration of host feeding by the parasitoid Aphelinus asychis [Hymenoptera: Eulophidae]. Entomophaga 19:479–482
Collier TR (1995) Host feeding, egg maturation, resorption, and longevity in the parasitoid Aphytis melinus (Hymenoptera: Aphelinidae). Ann Entomol Soc Am 88:206–2014
Dieckhoff C, Theobald JC, Wäckers FL, Heimpel GE (2014) Egg load dynamics and the risk of egg and time limitation experienced by an aphid parasitoid in the field. Ecol Evol 4:1739–1750
Eijs IEM, Ellers J, van Duinen GJ (1998) Feeding strategies in drosophilid parasitoids: the impact of natural food resources on energy reserves in females. Ecol Entomol 23:133–138
Ellers J (1996) Fat and eggs: an alternative method to measure the trade-off between survival and reproduction in insect parasitoids. Neth J Zool 46:227–235
Enkegaard A (1993) The poinsettia strain of the cotton whitefly, Bemisia tabaci (Homoptera: Aleyrodidae), biological and demographic parameters on poinsettia (Euphorbia pulcherrima) in relation to temperature. Bull Entomol Res 83:535–546
Frewin AJ, Xue Y, Welsman JA, Broadbent AB, Schaafsma AW, Hallett RH (2010) Development and parasitism by Aphelinus certus (Hymenoptera: Aphelinidae), a parasitoid of Aphis glycines (Hemiptera: Aphididae). Environ Entomol 39:1570–1578
Giron D, Rivero A, Mandon N, Darrouzet E, Casas J (2002) The physiology of host feeding in parasitic wasps: implications for survival. Funct Ecol 16:750–757
Giron D, Pincebourde S, Casas J (2004) Lifetime gains of host-feeding in a synovigenic parasitic wasp. Physiol Entomol 29:436–442
Hayes EJ, Wall R (1999) Age-grading adult-insects: a review of techniques. Physiol Entomol 24:1–10
Heimpel GE, Collier TR (1996) The evolution of host-feeding behaviour in insect parasitoids. Biol Rev 71:373–400
Heimpel GE, Mills NJ (2017) Biological control: ecology and applications. Cambridge University Press, Cambridge
Heimpel GE, Rosenheim JA, Mangel M (1996) Egg limitation, host quality, and dynamic behavior by a parasitoid in the field. Ecology 77:2410–2420
Heimpel GE, Rosenheim JA, Kattari D (1997a) Adult feeding and lifetime reproductive success in the parasitoid Aphytis melinus. Entomol Exp Appl 83:305–315
Heimpel GE, Rosenheim JA, Mangel M (1997b) Predation on adult Aphytis parasitoids in the field. Oecologia 110:346–352
Henderson PA, Southwood TRE (2016) Ecological methods, 4th edn. Wiley, Hoboken
Hopper KR, Prager SM, Heimpel GE (2013) Is parasitoids acceptance of different host species dynamics? Funct Ecol 27:1201–1211
Jervis MA, Kidd NAC (1986) Host-feeding strategies in hymenopteran parasitoids. Biol Rev 61:395–434
Jervis MA, Kidd NAC, Fitton MG, Huddleston T, Dawah HA (1993) Flower-visiting by hymenopteran parasitoids. J Nat Hist 27:67–105
Kidd NAC, Jervis MA (1989) The effects of host-feeding behaviour on the dynamics of parasitoid–host interactions, and the implications for biological control. Res Popul Ecol 31:235–274
Kidd NAC, Jervis MA (1991) Host-feeding and oviposition strategies of parasitoids in relation to host stage. Res Popul Ecol 33:13–28
Křiven V, Sirot E (1997) Searching for food or hosts: the influence of parasitoids behavior on host–parasitoid dynamics. Theor Popul Biol 51:201–209
Lee JC (2004) Diversifying agroecosystems with floral habitat to improve biological control [PhD Thesis]. University of Minnesota, Saint Paul
Lee JC, Heimpel GE (2008) Floral resources impact longevity and oviposition rate of a parasitoid in the field. J Anim Ecol 77:565–572
Lee JC, Leibee GL, Heimpel GE (2006) Broken wing fringe setae as a relative estimate of parasitoid age. Entomol Exp Appl 121:87–92
Líznarová E, Pekár S (2013) Dangerous prey is associated with a type 4 functional response in spiders. Anim Behav 85:1183–1190
Mahmoudi M, Sahragard A, Sendi JJ (2010) Effects of age and host availability on reproduction of Trioxys angelicae Haliday (Hymenoptera: Braconidae) parasitizing Aphis fabae Scopoli (Hemiptera: Aphididae). J Pest Sci 83:33–39
Michener CD, Cross EA, Daly HV, Rettenmeyer CW, Wille A (1955) Additional techniques for studying the behavior of wild bees (I). Insectes Soc 2:237–246
Miksanek JR, Heimpel GE (2019) A matrix model describing host–parasitoid population dynamics: the case of Aphelinus certus and soybean aphid. PLoS ONE 14:e0218217
Olson DM, Fadamiro H, Lundgren JG, Heimpel GE (2000) Effects of sugar feeding on carbohydrate and lipid metabolism in a parasitoid wasp. Physiol Entomol 25:17–26
Rivero-Lynch AP, Godfray HCJ (1997) The dynamics of egg production, oviposition and resorption in a parasitoid wasp. Funct Ecol 11:184–188
Röhne O (2002) Effect of temperature and host stage on performance of Aphelinus varipes Förster (Hym., Aphelinidae) parasitizing the cotton aphid, Aphis gossypii Glover (Hom., Aphididae). J Appl Entomol 126:572–576
Sahragard A, Jervis MA, Kidd NAC (1991) Influence of host availability on rates of oviposition and host-feeding, and on longevity in Dicondylus indianus Olmi (Hym., Dryinidae), a parasitoid of the Rice Brown Planthopper, Nilaparvata lugens Stål (Hem., Delphacidae). J Appl Entomol 112:153–162
Siekmann G, Tenhumberg B, Keller MA (2001) Feeding and survival in parasitic wasps: sugar concentration and timing matter. Oikos 95:425–430
Southwood TRE (1978) Ecological methods, 2nd edn. Chapman and Hall, London
Tena A, Senft M, Desneux N, Dregni J, Heimpel GE (2018) The influence of aphid-produced honeydew on parasitoid fitness and nutritional state: a comparative study. Basic Appl Ecol 29:55–68
Wäckers FL, van Rijn PCJ, Heimpel GE (2008) Honeydew as a food source for natural enemies: making the best of a bad meal? Biol Control 45:176–184
Watt WB, Chew FS, Snyder LRG, Watt AG, Rothschild DE (1977) Population structure of pierid butterflies. Oecologia 27:1–22
Weisser WW, Völkl W, Hassell MP (1997) The importance of adverse weather conditions for behaviour and population ecology of an aphid parasitoid. J Anim Ecol 66:386–400
Wu Z, Heimpel GE (2007) Dynamic egg maturation strategies in an aphid parasitoid. Physiol Entomol 32:143–149
Xu L, Zhou C, Xiao Y, Zhang P, Tang Y, Xu Y (2012) Insect oviposition plasticity in response to host availability: the case of the tephritid fruit fly Bactrocera dorsalis. Ecol Entomol 37:446–452
Yashima K, Murai T (2013) Development and reproduction of a potential biological control agent, Aphelinus varipes (Hymenoptera: Aphelinidae), at different temperatures. Appl Entomol Zool 48:21–26
Acknowledgements
We would like to thank Robert Koch, Robert Venette, and two anonymous reviewers for providing feedback on the manuscript as well as Jonathan Dregni and Komala Kanagala for maintaining laboratory colonies of Aphelinus certus and soybean aphid. This work was funded by a grant to GEH from the Minnesota Invasive Terrestrial Plants and Pests Center (MITPPC) through the Environment and Natural Resources Trust Fund, and JRM was supported in part by a Hueg-Harrison Fellowship.
Author information
Authors and Affiliations
Contributions
JRM and GEH conceived the study. JRM performed the experiments, analyzed the data, and drafted the manuscript. JRM and GEH edited the final manuscript.
Corresponding author
Additional information
Communicated by Heloise Gibb.
This study involves a simple approach to modeling survivorship and establishes an index of parasitoid age that may be applied to gain insight into the demographic trends of parasitoid populations.
Rights and permissions
About this article
Cite this article
Miksanek, J.R., Heimpel, G.E. Density-dependent lifespan and estimation of life expectancy for a parasitoid with implications for population dynamics. Oecologia 194, 311–320 (2020). https://doi.org/10.1007/s00442-020-04709-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04709-6