Abstract
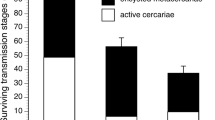
Free-living parasite infectious stages, such as the cercariae of trematodes (flatworms), can represent substantial biomass in aquatic ecosystems, yet their interactions with other planktonic fauna are poorly understood. Given that cercariae are consumed by various aquatic predators, sometimes even preferentially over zooplankton, their presence may decrease predation pressure on free-living organisms within similar trophic niches by serving as alternate prey. Here, we experimentally examined how the presence of cercariae (Plagiorchis sp.) affected the population dynamics of common freshwater zooplankton (Daphnia sp.) in the presence of a predator (the larval dragonfly, Leucorrhinia intacta) known to consume both. After seeding 48 mesocosms with starting populations of Daphnia, we used four treatments (12 replicates each) representing a factorial combination of the absence/presence of both cercariae and dragonfly larvae and tracked Daphnia populations over 4 weeks. We found a significant interaction between the presence of cercariae and predators on Daphnia population size. When faced with predation pressure, Daphnia reached ~ 50% higher numbers when accompanied by cercariae than without, suggesting a “protective” effect of the latter by acting as substitute prey. Within aquatic ecosystems, an abundance of trematodes may prove advantageous for zooplankton communities that share common predators, but further studies will be needed to determine how this varies depending on the predator, trematode, and zooplankton taxa involved.


Similar content being viewed by others
References
Akre BG, Johnson DM (1979) Switching and sigmoid functional response curves by damselfly naiads with alternative prey available. J Anim Ecol 48:703–720
Andersen T, Hessen DO (1991) Carbon, nitrogen, and phosphorus content of freshwater zooplankton. Limnol Oceanogr 36(4):807–814
Bernot RJ, Poulin R (2018) Ecological stoichiometry for parasitologists. Trends Parasitol 34:928–933
Blankespoor H (1970) Host-parasite relationships of an avian trematode, Plagiorchis noblei Park, 1936. PhD Dissertation. Iowa State University Library. http://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=5816&context=rtd. Accessed 5 May 2019
Blankespoor HD (1977) Notes on the biology of Plagiorchis noblei Park, 1936 (Trematoda: Plagiorchiidae). Proc Helminthol Soc Wash 44:44–50
Brett MT, Goldman CR (1997) Consumer versus resource control in freshwater pelagic food webs. Science 275:384–386
Brett MT, Muller-Navarra DC, Ballantyne AP, Ravet JL, Goldman CR (2006) Daphnia fatty acid composition reflects that of their diet. Limnol Oceanogr 51:2428–2437
Burks RL, Jeppesen E, Lodge DM (2001) Pelagic prey and benthic predators: impact of odonate predation on Daphnia. J N Am Benthol Soc 20:615–628
Burns CW (1992) Population dynamics of crustacean zooplankton in a mesotrophic lake, with emphasis on Boeckella hamata BREHM (Copepoda: Calanoida). Int Rev Gesamten Hydrobiol 77:553–577
Carpenter SR, Kitchell JF, Hodgson JR (1985) Cascading trophic interactions and lake productivity. Bioscience 35:634–639
Catania SVL, Koprivnikar J, McCauley SJ (2016) Size-dependent predation alters interactions between parasites and predators. Can J Zool 94:631–635
Christensen NO (1979) Schistosoma mansoni: interference with cercarial host-finding by various aquatic organisms. J Helminthol 53:7–14
Culver DA, Boucherle MM, Bean DJ, Fletcher JW (1985) Biomass of freshwater crustacean zooplankton from length–weight regressions. Can J Fish Aquat Sci 42:1380–1390
Dempster SJ, Rau ME (1990) The effects of single exposures of Aedes aegypti larvae and pupae to Plagiorchis noblei cercariae in the laboratory. J Parasitol 76:307–309
Dobson A, Lafferty KD, Kuris AM, Hechinger RF, Jetz W (2008) Homage to Linnaeus: how many parasites? How many hosts? PNAS 105:11482–11489
Dumont HJ, Van de Velde I, Dumont S (1975) The dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 19:75–97
Dunne JA, Lafferty KD, Dobson AP, Hechinger RF, Kuris AM, Martinez ND, McLaughlin JP, Mouritsen KN, Poulin R, Reise K, Stouffer DB (2013) Parasites affect food web structure primarily through increased diversity and complexity. PLoS Biol 11:e1001579
Ebert D (2005) Ecology, epidemiology, and evolution of parasitism in Daphnia. National Center for Biotechnology Information, Bethesda, Md. http://www.ncbi.nlm.nih.gov/books/NBK2042/
Esch GW, Barger MA, Fellis KJ (2002) The transmission of digenetic trematodes: style, elegance, complexity. Integr Comp Biol 42:304–312
Faltýnková A (2005) Larval trematodes (Digenea) in molluscs from small water bodies near Šeské Budšjovice, Czech Republic. Acta Parasitol 50:49–55
Fels D, Lee A, Ebert D (2004) The impact of microparasites on the vertical distribution of Daphnia magna. Arch Hydrobiol 161:65–80
Ferrari MC, Messier F, Chivers DP (2008) Can prey exhibit threat-sensitive generalization of predator recognition? Extending the predator recognition continuum hypothesis. Proc R Soc Lond B Biol Sci 275:1811–1816
Gliwicz ZM, Pijanowska J (1989) The role of predation in zooplankton succession. In: Plankton ecology. Springer, Berlin, pp 253–296
Haas W (1994) Physiological analyses of host-finding behavior in trematode cercariae—adaptations for transmission success. Parasitology 109:S15–S29
Hansen PJ, Bjørnsen PK (1997) Zooplankton grazing and growth: scaling within the 2–2,000-~µm body size range. Limnol Oceanogr 42:687–704
Hanson JM, Peters RH (1984) Empirical prediction of crustacean zooplankton biomass and profundal macrobenthos biomass in lakes. Can J Fish Aquat Sci 41:439–445
Hays GC (2002) A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 503:163–170
Hülsmann S, Weiler W (2000) Adult, not juvenile mortality as a major reason for the midsummer decline of a Daphnia population. J Plankton Res 22:151–168
Hunt RJ, Swift M (2010) Predation by larval damselflies on cladocerans. J Freshw Ecol 25:345–351
Jeffries M (1988) Individual vulnerability to predation: the effect of alternative prey types. Freshwater Biol 19:49–56
Johnson PT, Chase JM, Dosch KL, Hartson RB, Gross JA, Larson DJ, Sutherland DR, Carpenter SR (2007) Aquatic eutrophication promotes pathogenic infection in amphibians. PNAS 104:15781–15786
Johnson PTJ, Dobson A, Lafferty KD, Marcogliese DJ, Memmott J, Orlofske SA, Poulin R, Thieltges DW (2010) When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol Evol 25:362–371
Johnson PTJ, Stanton DE, Forshay KJ, Calhoun DM (2018) Vertically challenged: how disease suppresses Daphnia vertical migration behavior. Limnol Oceanogr 63:886–896
Johnston TH, Angel LM (1951) The life history of Plagiorchis jaenschi, a new trematode from the Australian water rat. Trans R Soc S Aust 74:49–58
Koprivnikar J, Penalva L (2015) Lesser of two evils? Foraging choices in response to threats of predation and parasitism. PLoS One 10:e0116569
Koprivnikar J, Urichuk TMY, Szuroczki D (2017) Influences of habitat and arthropod density on parasitism in two co-occurring host taxa. Can J Zool 95:589–597
Koprivnikar J, Riepe TB, Calhoun DM, Johnson PT (2018) Whether larval amphibians school does not affect the parasite aggregation rule: testing the effects of host spatial heterogeneity in field and experimental studies. Oikos 127:99–110
Kuris AM, Hechinger RF, Shaw JC, Whitney KL, Aguirre-Macedo L, Boch CA, Dobson AP, Dunham EJ, Fredensborg BL, Huspeni TC, Lorda J, Mababa L, Mancini FT, Mora AB, Pickering M, Talhouk NL, Torchin ME, Lafferty KD (2008) Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454:515–518
Lafferty KD, Allesina S, Arim M, Briggs CJ, De Leo G, Dobson P, Dunne JA, Johnson PTJ, Kuris AM, Marcogliese DJ, Martinez ND, Memmott J, Marquet PA, McLaughlin JP, Mordecai EA, Pascual M, Poulin R, Thieltges DW (2008) Parasites in food webs: the ultimate missing links. Ecol Lett 11:533–546
Lo CT, Lee KM (1996) Pattern of emergence and the effects of temperature and light on the emergence and survival of heterophyid cercariae (Centrocestus formosanus and Haplorchis pumilio). J Parasitol 82:347–350
Loy C, Haas W (2001) Prevalence of cercariae from Lymnaea stagnalis snails in a pond system in southern Germany. J Parasitol Res 87:878–882
Lynch M (1979) Predation, competition, and zooplankton community structure: an experimental study. Limnol Oceanog 24:253–272
Maloney CL, Field JG (1991) The size-based dynamics of plankton food webs. I. A simulation model of carbon and nitrogen flows. J Plankton Res 13:1003–1038
Marcogliese DJ, Cone DK (1997) Food webs: a plea for parasites. Trends Ecol Evol 12:320–325
Marsit CJ, Fried B, Sherma J (2000) Neutral lipids in cercariae, encysted metacercariae, and rediae of Echinostoma caproni. J Helminthol 74:365–367
McCarthy AM (1999) Photoperiodic cercarial emergence patterns of the digeneans Echinoparyphium recurvatum and Plagiorchis sp. from a mixed infection in Lymnaea peregra. J Helminthol 73:59–62
Mironova E, Gopko M, Pasternak A, Mikheev V, Taskinen J (2019) Trematode cercariae as prey for zooplankton: effect on fitness traits of predators. Parasitology 146:105–111
Morley NJ (2012) Cercariae (Platyhelminthes: Trematoda) as neglected components of zooplankton communities in freshwater habitats. Hydrobiologia 691:7–19
Morley NJ, Lewis JW (2013) Thermodynamics of cercarial development and emergence in trematodes. Parasitology 140:1211–1224
Mouritsen KN, Poulin R (2002) Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitology 124:S101–S117
Orlofske SA, Jadin RC, Preston DL, Johnson PT (2012) Parasite transmission in complex communities: predators and alternative hosts alter pathogenic infections in amphibians. Ecology 93:1247–1253
Orlofske SA, Jadin RC, Johnson PTJ (2015) It’s a predator-eat-parasite world: how characteristics of predator, parasite and environment affect consumption. Oecologia 178:537–547
Pace ML, Orcutt JD (1981) The relative importance of protozoans, rotifers, and crustaceans in a freshwater zooplankton community. Limnol Oceanogr 26:822–830
Poulin R (2006) Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 132:143–151
Preston DL, Orlofske SA, Lambden JP, Johnson PTJ (2013) Biomass and productivity of trematode parasites in pond ecosystems. J Anim Ecol 82:509–517
Rohr JR, Civitello DJ, Crumrine PW, Halstead NT, Miller AD, Schottheofer AM, Stenoien C, Johnson LB, Beasley VR (2015) Predator diversity, intraguild predation, and indirect effects drive parasite transmission. PNAS 112:3008–3013
Schell SC (1985) Handbook of trematodes of North America north of Mexico. University of Idaho Press, Moscow
Schotthoefer AM, Labak KM, Beasley VR (2007) Ribeiroia ondatrae cercariae are consumed by aquatic invertebrate predators. J Parasitol 93(5):1240–1243
Schriver P, Bøgestrond J, Jeppeson E, Søndergaard M (1995) Impact of submerged macrophytes on fish-zooplankton-phytoplankton interactions: large-scale enclosure experiments in a shallow eutrophic lake. Freshw Biol 33:255–270
Schwartz SS, Cameron GN (1993) How do parasites cost their hosts? Preliminary answers from trematodes and Daphnia obtusa. Limnol Oceanogr 38:602–612
Soldánová M, Selbach C, Sures B (2016) The early worm catches the bird? Productivity and patterns of Trichobilharzia szidati cercarial emission from Lymnaea stagnalis. PLoS One 11:e0149678
Szuroczki D, Richardson JM (2009) The role of trematode parasites in larval anuran communities: an aquatic ecologist’s guide to the major players. Oecologia 161:371–385
Thieltges DW, de Montaudouin X, Fredensborg B, Jensen KT, Koprivnikar J, Poulin R (2008) Production of marine trematode cercariae: a potentially overlooked path of energy flow in benthic systems. Mar Ecol Prog Ser 372:147–155
Thieltges DW, Amundsen PA, Hechinger RF, Johnson PT, Lafferty KD, Mouritsen KN, Preston DL, Reise K, Zander CD, Poulin R (2013) Parasites as prey in aquatic food webs: implications for predator infection and parasite transmission. Oikos 122:1473–1482
Vielma S, Lagrue C, Poulin R, Selbach C (2019) Non-host organisms impact transmission at two different life stages in a marine parasite. Parasitol Res 118:111–117
Wagenbach GE, Alldredge AL (1974) Effect of light on the emergence pattern of Plagiorchis micracanthos cercariae from Stagnicola exilis. J Parasitol 60:782–785
Watertor JL (1965) Intraspecific variation among trematodes of the genus Telorchis. Ph.D. Dissertation. Iowa State University Library. https://doi.org/10.31274/rtd-180813-416
Webber RA, Rau ME, Lewis DJ (1986) The effects of various light regimens on the emergence of Plagiorchis noblei cercariae from the molluscan intermediate host, Stagnicola elodes. J Parasitol 72:703–705
Weinstein SB, Moura CW, Mendez JF, Lafferty KD (2018) Fear of feces? Tradeoffs between disease risk and foraging drive animal activity around raccoon latrines. Oikos 127:927–934
Welsh JE, Liddell C, Van Der Meer J, Thieltges DW (2017) Parasites as prey: the effect of cercarial density and alternative prey on consumption of cercariae by four non-host species. Parasitology 144:1775–1782
Wissinger SA (1988) Spatial distribution, life history and estimates of survivorship in a fourteen-species assemblage of larval dragon-flies (Odonata: Anisoptera). Freshw Biol 20:29–340
Wojdak JM, Edman RM, Wyderko JA, Zemmer SA, Belden LK (2014) Host density and competency determine the effects of host diversity on trematode parasite infection. PLoS One 9:e105059
Wu L, Culver DA (1994) Daphnia population dynamics in western Lake Erie: regulation by food limitation and yellow perch predation. J Great Lakes Res 20:537–545
Yoder HR, Coggins JR (1998) Larval trematode assemblages in the snail Lymnaea stagnalis from southeastern Wisconsin. J Parasitol 84:259–268
Zakikhani M, Rau ME (1999) Plagiorchis elegans (Digenea: Plagiorchiidae) infections in Stagnicola elodes (Pulmonata: Lymnaeidae): Host susceptibility, growth, reproduction, mortality, and cercarial production. J Parasitol 85:454–463
Acknowledgements
We thank L. Santos and J. Nguyen for experimental assistance, as well as S. J. McCauley for advice. This work was supported by an NSERC Discovery grant to J. K. (RGPIN-2015-05566).
Author information
Authors and Affiliations
Contributions
BS and JK conceived and designed the experiments. BS performed the experiments. BS and JK analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Pieter Johnson.
Here we demonstrate that free-living parasite infectious stages interact with other zooplankton and “protect” these by serving as alternative prey, with potentially broad effects of their consumption.
Rights and permissions
About this article
Cite this article
Schultz, B., Koprivnikar, J. Free-living parasite infectious stages promote zooplankton abundance under the risk of predation. Oecologia 191, 411–420 (2019). https://doi.org/10.1007/s00442-019-04503-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04503-z