Abstract
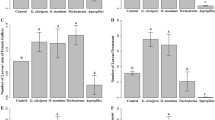
Plant pathogens can have cascading consequences on insect herbivores, though whether they alter competition among resource-sharing insect herbivores is unknown. We experimentally tested whether the infection of a plant pathogen, the parasitic plant dwarf mistletoe (Arceuthobium americanum), on jack pine (Pinus banksiana) altered the competitive interactions among two groups of beetles sharing the same resources: wood-boring beetles (Coleoptera: Cerambycidae) and the invasive mountain pine beetle (Dendroctonus ponderosae) (Coleoptera: Curculionidae). We were particularly interested in identifying potential mechanisms governing the direction of interactions (from competition to facilitation) between the two beetle groups. At the lowest and highest disease severity, wood-boring beetles increased their consumption rate relative to feeding levels at moderate severity. The performance (brood production and feeding) of mountain pine beetle was negatively associated with wood-boring beetle feeding and disease severity when they were reared separately. However, when both wood-boring beetles and high severity of plant pathogen infection occurred together, mountain pine beetle escaped from competition and improved its performance (increased brood production and feeding). Species-specific responses to changes in tree defense compounds and quality of resources (available phloem) were likely mechanisms driving this change of interactions between the two beetle groups. This is the first study demonstrating that a parasitic plant can be an important force in mediating competition among resource-sharing subcortical insect herbivores.






Similar content being viewed by others
References
Adams AS, Six DL (2007) Temporal variation in mycophagy and prevalence of fungi associated with developmental stages of Dendroctonus ponderosae (Coleoptera: Curculionidae). Environ Entomol 36:64–72
Adams AS, Boone CK, Bohlmann J, Raffa KF (2011) Responses of bark beetle-associated bacteria to host monoterpenes and their relationship to insect life history. J Chem Ecol 37:808–817
Adams AS, Aylward FO, Adams SM, Erbilgin N, Aukema B, Currie CR, Suen G, Raffa KF (2013) Mountain pine beetles colonizing historical and naïve host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Appl Environ Microbiol 79:3468–3475
Allison JD, Borden JH, McIntosh RL, de Groot P, Gries G (2001) Kairomonal response by four Monochamus species (Coleoptera: Cerambycidae) to bark beetle pheromones. J Chem Ecol 27:633–646
Altieri AH, van Wesenbeeck BK, Bertness MD, Silliman BR (2010) Facilitation cascade drives positive relationship between native biodiversity and invasion success. Ecology 91:1269–1275
Amman GD (1972) Mountain pine beetle brood production in relation to thickness of lodgepole pine phloem. J Econ Entomol 65:138–140
Amman GD, Cole WE (1983) Mountain pine beetle dynamics in lodgepole pine forests. Part II. Population dynamics. General technical report INT-145. USDA Forest Service, Intermountain Forest and Range Experiment Station, Ogden, UT
Armas C, Ordiales R, Pugnaire FI (2004) Measuring plant interactions: a new comparative index. Ecology 85:2682–2686
Barrio IC, Hik DS, Bueno CG, Cahill JF (2013) Extending the stress-gradient hypothesis—is competition among animals less common in harsh environments? Oikos 122:516–523
Berner D, Blanckenhorn WU, Korner C (2005) Grasshoppers cope with low host plant quality by compensatory feeding and food selection: N limitation challenged. Oikos 111:525–533
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Bleiker KP, Six DL (2007) Dietary benefits of fungal associates to an eruptive herbivore: potential implications of multiple associates on host population dynamics. Environ Entomol 36:1384–1396
Bonello P, Gordon TR, Herms DA, Wood DL, Erbilgin N (2006) Nature and ecological implications of pathogen-induced systemic resistance in conifers: a novel hypothesis. Physiol Mol Plant Pathol 68:95–104
Colgan LJ, Erbilgin N (2011) Tree-mediated interactions between the jack pine budworm and a mountain pine beetle fungal associate. Ecol Entomol 36:425–434
Cullingham CI, Cooke JEK, Dang S, Davis CS, Cooke BJ, Coltman DW (2011) Mountain pine beetle host-range expansion threatens the boreal forest. Mol Ecol 20:2157–2171
Dangles O, Herrera M, Anthelme F (2013) Experimental support of the stress-gradient hypothesis in herbivore-herbivore interaction. New Phytol 197:405–408
DiGuistini S, Ralph SG, Lim YW, Holt R, Jones S, Bohlmann J, Breuil C (2007) Generation and annotation of lodgepole pine and oleoresin-induced expressed sequences from the blue-stain fungus Ophiostoma clavigerum, a mountain pine beetle-associated pathogen. FEMS Microbiol Lett 267:151–158
Dodds KJ, Graber C, Stephen FM (2001) Facultative intraguild predation by larval Cerambycidae (Coleoptera) on bark beetle larvae (Coleoptera: Scolytidae). Environ Entomol 30:17–22
Dyer LJ, Seabrook WD (1978) Some aspects of oviposition site selection in Monchamus notatus and M. scutellatus (Coleoptera: Cerambycidae). J Chem Ecol 4:199–210
Erbilgin N, Colgan LJ (2012) Differential effects of plant ontogeny and damage type on phloem and foliage monoterpenes in jack pine (Pinus banksiana). Tree Physiol 32:946–957
Erbilgin N, Raffa KF (2001) Kairomonal range of generalist predators in specialized habitats: responses to multiple phloeophagous species emitting pheromones vs. host odors. Entomol Exp Appl 99:205–210
Erbilgin N, Christiansen E, Krokene P, Zeneli G, Gershenzon J (2006) Exogenous application of methyl jasmonate elicits defences in Norway spruce (Picea abies) and reduces host colonization by the bark beetle Ips typographus. Oecologia 148:426–436
Erbilgin N, Ma C, Whitehouse C, Shan B, Najar A, Evenden M (2014) Chemical similarity between historical and novel host plants promotes range and host expansion of the mountain pine beetle in a naïve host ecosystem. New Phytol 201:940–950
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defences of conifer bark against bark beetles and other pests. New Phytol 167:353–376
Furniss RL, Carolin VM (1977) Western forest insects. USDA For Serv Misc Publ 1339:654
Gardiner LM (1975) Deterioration of fire-killed pine in Ontario and the causal wood-boring beetles. Can Entomol 89:241–263
Goodsman DW, Erbilgin N, Lieffers VJ (2012) The impact of phloem nutrients on overwintering mountain pine beetles and their fungal symbionts. Environ Entomol 41:478–486
Goodsman DW, Lusebrink I, Landhausser SM, Erbilgin N, Lieffers VJ (2013) Variation in carbon availability, defence chemistry and susceptibility to fungal invasion along the stems of mature trees. New Phytol 197:586–594
Hawksworth FG, Wiens D (1996) Dwarf mistletoes: biology, pathology and systematics. Agriculture handbook 709. USDA Forest Service, Washington, DC, p 410
Johnson DW, Yarger LC, Minnemeyer CD, Pace VE (1976) Dwarf mistletoe as a predisposing factor for mountain pine beetle attack in ponderosa pine in Colorado front range. Forest insect and disease biological evaluation R2-4, USDA Forest Service, Rocky Mountain Region, p 7
Kant MR, Jonckheere W, Knegt B, Lemos F, Liu J, Schimmel BCJ, Villarroel CA, Ataide LMS, Dermauw W, Glas JJ, Egas M, Janssen A, Van Leeuwen T, Schuurink RC, Sabelis MW, Alba JM (2015) Mechanisms and ecological consequences of plant defense induction and suppression in herbivore communities. Ann Bot 115:1015–1051
Karban R, Grof-Tisza P, Holyoak M (2012) Facilitation of tiger moths by outbreaking tussock moths that share the same host plants. J Anim Ecol 81:1095–1102
Keeling CI, Bohlmann J (2006) Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence on conifers against insects and pathogens. New Phytol 170:657–675
Kenaley SNC, Mathiasen RL, Daugherty CM (2006) Selection of dwarf mistletoe-infected ponderosa pines by Ips species (Coleoptera: Scolytidae) in northern Arizona. West N Am Nat 66:279–284
Langenheim JH (1994) Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol 20:1223–1280
Lawton JH, Strong DR (1981) Community patterns and competition in folivorous insects. Am Nat 118:317–338
Levin SA (1970) Community equilibria and stability, and an extension of the competitive exclusion principle. Am Nat 104:413–423
Liebhold AM, Tobin PC (2008) Population ecology of insect invasions and their management. Annu Rev Entomol 53:387–408
Lusebrink I, Evenden ML, Guillaume Blanchet F, Cooke JEK, Erbilgin N (2011) Effect of water stress and fungal inoculation on monoterpene emission from an historical and a new pine host of the mountain pine beetle. J Chem Ecol 37:1013–1026
Maestre FT, Callaway RM, Valladares F, Lortie CJ (2009) Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J Ecol 97:199–205
Mattson WJ, Haack RA (1987) Role of drought in outbreaks of plant-eating insects. BioScience 37:110–118
Nebeker TE, Schmitz RF, Tisdale RA, Hobson KR (1995) Chemical and nutritional status of dwarf mistletoe, Armillaria root rot, and Comandra blister rust infected trees which may influence tree susceptibility to bark beetle attack. Can J Bot 73:360–369
Paine TD, Raffa KF, Harrington TC (1997) Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42:178–206
Peddle S, de Groot P, Smith S (2002) Oviposition behavior and response of Monochamus scutellatus (Coleoptera: Cerambycidae) to conspecific eggs and larvae. Agric For Entomol 4:217–222
Raffa KF, Powell EN, Townsend PA (2013) Temperature-driven range expansion of an irruptive insect heightened by weakly coevolved plant defences. Proc Natl Acad Sci USA 110:2193–2198
Röder G, Rahier M, Naisbit RE (2007) Coping with an antagonist: the impact of a phytopathogenic fungus on the development and behavior of two species of alpine leaf beetle. Oikos 116:1514–1523
Safranyik L, Carroll AL (2006) The biology and epidemiology of mountain pine beetle in lodgepole pine forests, chapter 1. In: Safranyik L, Wilson B (eds) The mountain pine beetle a synthesis of biology, management, and impacts on lodgepole pine. Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre, Victoria, pp 3–66
Safranyik L, Shore TL, Linton DA, Rankin L (1999) Effects of induced competitive interactions with secondary bark beetle species on establishment and survival of mountain pine beetle broods in lodgepole pine. Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre, BC-X-384, p 33
Safranyik L, Carroll AL, Regniere J, Langor DW, Riel WG, Shore TL, Peter B, Cooke BJ, Nealis VG, Taylor SW (2010) Potential for range expansion of mountain pine beetle into the boreal forest of North America. Can Entomol 142:415–442
Sanchez-Husillos E, Álvarez-Bas G, Etxebeste I, Pajares JA (2013) Shoot feeding, oviposition, and development of Monochamus galloprovincialis on Pinus pinea relative to other pine species. Entomol Exp Appl 149:1–10
Smith GD, Carroll AL, Lindgren BS (2011) Facilitation in bark beetles: endemic mountain pine beetle gets a helping hand. Agric For Entomol 13:37–43
Stout MJ, Thaler JS, Thomma BPHJ (2006) Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu Rev Entomol 51:663–689
Tack AJM, Dicke M (2013) Plant pathogens structure arthropod communities across multiple spatial and temporal scales. Funct Ecol 27:633–645
Tack AJM, Gripenberg S, Roslin T (2012) Cross-kingdom interactions matter: fungal-mediated interactions structure an insect community on oak. Ecol Lett 15:177–185
Therrien J, Mason CJ, Cale JA, Adams A, Aukema BH, Currie CR, Raffa KF, Erbilgin N (2015) Bacteria influence mountain pine beetle brood development through interactions with symbiotic and antagonistic fungi: implications for climate-driven host range expansion. Oecologia 179:467–485
Zargaran MR, Erbilgin N, Ghosta Y (2012) Changes in oak gall wasps species diversity (Hymenoptera: Cynipidae) in relation to the presence of oak powdery mildew (Erysiphe alphitoides). Zool Stud 51:175–184
Acknowledgments
Funding for this research was provided by the Alberta Innovates–New Faculty Award, Canada Research Chairs program, and NSERC Discovery to N. E., as well as Alberta Innovates-Technology Futures, the Vanier Canada Graduate Scholarship, and the Izzak Walton Killam Memorial Scholarship to J. G. K. L. Barnhardt and D. Letourneau from Alberta Environment and Sustainable Resource Development helped with site selection. S. Taft, A. Sturm, I. Lusebrink and J. Therrien contributed to field experiments. We would like to thank J. F. Cahill, J. Karst, C. Tabacaru (University of Alberta) and two anonymous reviewers for valuable inputs that greatly improved the manuscript. The authors declare that they have no conflict of interest.
Author contribution statement
J. G. K. and N. E. conceived and designed the research experiments and wrote the paper; J. G. K. and A. N. conducted the chemical analysis; J. G. K. and J. A. C. analyzed the data; all authors provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Diethart Matthies.
Highlighted Student Paper Justification: Plant pathogens can have cascading consequences on resource-sharing insect herbivores, though whether they can alter interspecies interactions (competition, facilitation) is unknown. As part of the JGK's PhD research, the results show that the competitive effect of native wood-boring beetles on a non-native invasive bark beetle was dependent on plant pathogeninduced changes in host tree quality and chemical defenses. Identifying the impact of tree-mediated interactions on community dynamics of herbivores can be critical to understanding and predicting invasive species establishment success and population dynamics in novel habitats.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Klutsch, J.G., Najar, A., Cale, J.A. et al. Direction of interaction between mountain pine beetle (Dendroctonus ponderosae) and resource-sharing wood-boring beetles depends on plant parasite infection. Oecologia 182, 1–12 (2016). https://doi.org/10.1007/s00442-016-3559-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3559-8