Abstract
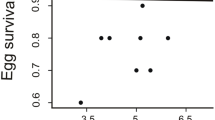
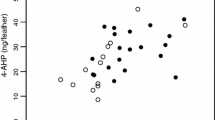
Much of the debate surrounding the selective forces responsible for the expression of conspicuous plumage colouration is centred on the question of precisely which individual qualities are signalled by carotenoid- and melanin-based pigments. To examine this and other related issues, we performed viability selection analyses in wild-caught captive male greenfinches (Carduelis chloris) in Estonia during winters between 2003 and 2014. Based on our measurements, birds with a darker black eumelanin-based colouration of tail feathers survived better than those whose tail feathers had a paler black colouration. The carotenoid-based yellow colouration of the same feathers was not associated with mortality in captivity and showed much less between-year variation in the field than the black colouration. Between year-variation in the black (but not yellow) colouration of feathers was parallel in wild-grown feathers (on birds in the wild) and laboratory-grown ones (on birds held temporarily in captivity). Taken together, these findings imply that eumelanotic colouration in greenfinches is currently under selection and suggest the presence of sufficient genetic variation for a rapid response to selection. In particular, tail feathers have become darker black since the emergence of avian trichomonosis, which is known to selectively kill paler individuals.





Similar content being viewed by others
References
Atkinson CT, Thomas NJ, Hunter DB (2008) Parasitic diseases of wild birds. Wiley Online Library, Singapore
Badyaev AV, Hill GE (2000) Evolution of sexual dichromatism: contribution of carotenoid versus melanin-based coloration. Biol J Linn Soc 69:153–172
Badyaev AV, Young RL (2004) Complexity and integration in sexual ornamentation: an example with carotenoid and melanin plumage pigmentation. J Evol Biol 17:1317–1327
Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S (2010) The melanocortin system in control of inflammation. Sci World J 10:1840–1853
Chew BP, Park JS (2004) Carotenoid action on the immune response. J Nutr 134:S257–S261
Cramp P, Perrins CM (1994) The birds of western Palearctic. Oxford University Press, Oxford
Ducrest AL, Keller L, Roulin A (2008) Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol Evol 23:502–510. doi:10.1016/j.tree.2008.06.001
Ducrest A-L, Ursenbacher S, Golay P et al (2014) Pro-opiomelanocortin gene and melanin-based colour polymorphism in a reptile. Biol J Linn Soc 111:160–168. doi:10.1111/bij.12182
Dunn PO, Garvin JC, Whittingham LA, Freeman-Gallant CR, Hasselquist D (2010) Carotenoid and melanin-based ornaments signal similar aspects of male quality in two populations of the common yellowthroat. Funct Ecol 24:149–158
Emaresi G, Ducrest A-L, Bize P, Richter H, Simon C, Roulin A (2013) Pleiotropy in the melanocortin system: expression levels of this system are associated with melanogenesis and pigmentation in the tawny owl (Strix aluco). Mol Ecol 22:4915–4930. doi:10.1111/mec.12438
Emaresi G, Bize P, Altwegg R et al (2014) Melanin-specific life-history strategies. Am Nat 183:269–280. doi:10.1086/674444
Evans SR, Sheldon BC (2012) Pigments versus structure: examining the mechanism of age-dependent change in a carotenoid-based colour. J Anim Ecol 82:418–428. doi:10.1111/1365-2656.12008
Gangoso L, Grande JM, Ducrest AL et al (2011) MC1R-dependent, melanin-based colour polymorphism is associated with cell-mediated response in the Eleonora’s falcon. J Evol Biol 24:2055–2063. doi:10.1111/j.1420-9101.2011.02336.x
Giovannini S, Pewsner M, Hussy D et al (2013) Epidemic of salmonellosis in passerine birds in Switzerland with spillover to domestic cats. Vet Pathol 50:597–606. doi:10.1177/0300985812465328
Griffith SC, Parker TH, Olson VA (2006) Melanin-versus carotenoid-based sexual signals: is the difference really so black and red? Anim Behav 71:749–763
Guindre-Parker S, Love OP (2014) Revisiting the condition-dependence of melanin-based plumage. J Avian Biol 45:29–33. doi:10.1111/j.1600-048X.2013.00190.x
Hill GE (1992) Proximate basis of variation in carotenoid pigmentation in male House finches. Auk 109:1–12
Hill GE (2006) Female mate choice for ornamental colouration. In: Hill GE, McGraw KJ (eds) Bird colouration: function and evolution, vol 2. Harvard University Press, Cambridge, pp 137–200
Hill GE (2011) Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol Lett 14:625–634. doi:10.1111/j.1461-0248.2011.01622.x
Hill GE (2014) Cellular respiration: the nexus of stress, condition, and ornamentation. Integr Comp Biol 54:645–657. doi: 10.1093/icb/icu029
Hill GE, Johnson JD (2012) The vitamin A-redox hypothesis: a biochemical basis for honest signaling via carotenoid pigmentation. Am Nat 180:E127–E150
Hill GE, McGraw KJ (2006) Bird coloration: function and evolution, vol 2. Harvard University Press, Cambridge
Hõrak P, Sild E, Soomets U, Sepp T, Kilk K (2010) Oxidative stress and information content of black and yellow plumage coloration: an experiment with greenfinches. J Exp Biol 213:2225–2233. doi:10.1242/jeb.042085
Jawor JM, Breitwisch R (2003) Melanin ornaments, honesty, and sexual selection. Auk 120:249–265
Keyser AJ, Siefferman LM (2005) Viability selection against highly ornamented males. Evol Ecol Res 7:595
Kittilsen S, Johansen IB, Braastad BO, Øverli Ø (2012) Pigments, parasites and personalitiy: towards a unifying role for steroid hormones? PLoS One 7:e34281. doi:10.1371/journal.pone.0034281
Lawson B, Howard T, Kirkwood JK et al (2010) Epidemiology of salmonellosis in garden birds in England and wales, 1993–2003. Ecohealth 7:294–306
Lawson B, Robinson RA, Neimanis A et al (2011) Evidence of spread of the emerging infectious disease, finch trichomonosis, by migrating birds. Ecohealth 8:143–153. doi:10.1007/s10393-011-0696-8
Lehikoinen A, Lehikoinen E, Valkama J, Väisänen RA, Isomursu M (2013) Impacts of trichomonosis epidemics on greenfinch Chloris chloris and Chaffinch Fringilla coelebs populations in Finland. Ibis 155:357–366. doi:10.1111/ibi.12028
Lozano GA (1994) Carotenoids, parasites and sexual selection. Oikos 70:309–311
Männiste M, Hõrak P (2014) Emerging infectious disease selects for darker plumage coloration in greenfinches. Front Ecol Evol 2:4. doi: 10.3389/fevo.2014.00004
McBurney S, Kelly-Clark WK, Forzán MJ, Lawson B, Tyler KM, Greenwood SJ (2015) Molecular characterization of Trichomonas gallinae isolates recovered from the Canadian Maritime provinces’ wild avifauna reveals the presence of the genotype responsible for the European finch trichomonosis epidemic and additional strains. Parasitology 142:1053–1062. doi:10.1017/S0031182015000281
McGraw KJ (2008) An update on the honesty of melanin-based color signals in birds. Pigment Cell Melanoma Res 21:133–138. doi:10.1111/j.1755-148X.2008.00454.x
Møller AP, Erritzøe J (2000) Predation against birds with low immunocompetence. Oecologia 122:500–504
Møller AP, Biard C, Blount JD et al (2000) Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult Biol Rev 11:137–159
Moreno J, Møller A (2006) Are melanin ornaments signals of antioxidant and immune capacity in birds. Acta Zool Sin 52:202–208
Neimanis AS, Handeland K, Isomursu M et al (2010) First report of epizootic trichomoniasis in wild finches (family Fringillidae) in Southern Fennoscandia. Avian Dis 54:136–141
Newton I, Rothery P (2005) The timing, duration and pattern of moult and its relationship to breeding in a population of the European greenfinch Carduelis chloris. Ibis 147:667–679
Owens IPF, Hartley IR (1998) Sexual dimorphism in birds: why are there so many different forms of dimorphism? Proc R Soc Lond B Biol Sci 265:397–407
Peters A, Delhey K, Andersson S, van Noordwijk H, Forschler MI (2008) Condition-dependence of multiple carotenoid-based plumage traits: an experimental study. Funct Ecol 22:831–839. doi:10.1111/j.1365-2435.2008.01437.x
Piault R, Gasparini J, Bize P, Jenni-Eiermann S, Roulin A (2009) Pheomelanin-based coloration and the ability to cope with variation in food supply and parasitism. Am Nat 174:548–556
Poston JP, Hasselquist D, Stewart IRK, Westneat DF (2005) Dietary amino acids influence plumage traits and immune responses of male house sparrows, Passer domesticus, but not as expected. Anim Behav 70:1171–1181
Récapet C, Dauphin L, Jacquin L, Gasparini J, Prévot-Julliard A-C (2013) Eumelanin-based colouration reflects local survival of juvenile feral pigeons in an urban pigeon house. J Avian Biol 44:583–590. doi:10.1111/j.1600-048X.2013.00087.x
Robinson RA, Lawson B, Toms MP et al (2010) Emerging infectious disease leads to rapid population declines of common British birds. PLoS One 5:e12215. doi:10.1371/journal.pone.0012215
Roulin A (2004) The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol Rev 79:815–848. doi:10.1017/S1464793104006487
Roulin A (2015) Condition-dependence, pleiotropy and the handicap principle of sexual selection in melanin-based colouration. Biol Rev. doi:10.1111/brv.12171
Roulin A, Ducrest A-L (2013) Genetics of coloration in birds. Semin Cell Dev Biol 24:595–605
Roulin A, Riols C, Dijkstra C, Ducrest AL (2001) Female plumage spottiness signals parasite resistance in the barn owl (Tyto alba). Behav Ecol 12:103–110
Roulin A, Altwegg R, Jensen H, Steinsland I, Schaub M (2010) Sex-dependent selection on an autosomal melanic female ornament promotes the evolution of sex ratio bias. Ecol Lett 13:616–626. doi:10.1111/j.1461-0248.2010.01459.x
Saks L, McGraw K, Hõrak P (2003) How feather colour reflects its carotenoid content. Funct Ecol 17:555–561
Senar JC, Quesada J (2006) Absolute and relative signals: a comparison between melanin- and carotenoid-based patches. Behaviour 143:589–595
Simons MJP, Cohen AA, Verhulst S (2012) What does carotenoid-dependent coloration tell? Plasma carotenoid level signals immunocompetence and oxidative stress state in birds? A meta-analysis. PLoS One 7:e43088
Svensson L (1992) Identification guide to European passerines. British Trust for Ornithology, The Nunnery, Thetford
Svensson P, Wong B (2011) Carotenoid-based signals in behavioural ecology: a review. Behaviour 148:131–189
Tyczkowski JK, Schaeffer JL, Hamilton PB (1991) Measurment of malabsorption of carotenoids in chickens with pale-birds syndrome. Poult Sci 70:2275–2279
Vinkler M, Albrecht T (2009) Carotenoid maintenance handicap and the physiology of carotenoid-based signalisation of health. Naturwissenschaften 97:19–28
von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc Lond B Biol Sci 266:1–12
Acknowledgments
We thank Indrek Ots, Lauri Saks, Elin Sild, Ulvi Karu, Tuul Sepp, Richard Meitern and Mari-Ann Lind for the help with bird catching and maintenance, Toomas Tammaru for statistical advice, and Jaanis Lodjak for help with the graphics. T. Sepp, two anonymous reviewers and AE provided constructive criticism on the manuscript. R. Meitern helped with programming, and Olavi Vainu from Matsalu Ringing Centre kindly provided data on recoveries of Fennoscandian greenfinches. Mati Martinson was of invaluable help with catching the birds in Saaremaa. The study was financed by the Estonian Ministry of Education and Science (Target-Financing project #0180004s09 and Institutional Research grant IUT34-8) and by the European Union through the European Regional Development Fund (Centre of Excellence Frontiers in Biodiversity Research).
Author contribution statement
PH and MM conceived the study; MM performed the measurements of plumage colouration; PH & and MM analysed the data; PH wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Toni Laaksonen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hõrak, P., Männiste, M. Viability selection affects black but not yellow plumage colour in greenfinches. Oecologia 180, 23–32 (2016). https://doi.org/10.1007/s00442-015-3451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3451-y