Abstract
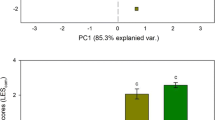
Stress factors may severely constrain the range of plant physiological responses in harsh environments. Convergence of traits is expected in coastal dunes because of environmental filtering imposed by severe abiotic factors. However, the wide range of morphological and phenological traits exhibited by coexisting dune species suggests considerable variation in functional traits. We hypothesized that the constraints imposed by structural traits ought to translate into physiological differences. Five dominant species with different morphological traits, but coexisting in a homogeneous dune area in Northwest Spain, were selected for study. Soil characteristics and leaf functional traits were measured in April, June and November 2008. Integrated water-use efficiency (assessed by C isotope discrimination) and N acquisition and use strategies (estimated by N isotope composition) varied significantly among species and the differences changed over time. Species differences in specific leaf area, relative water content, leaf N and C:N ratio, also varied over time. The species differed in stomatal density but not in soil characteristics, with the exception of pH. Species differences in functional traits related to the use of resources suggest species niche segregation. Species-specific temporal effects on the use of these resources support temporal niche differentiation. Somewhat in contrast to the findings of previous studies on harsh environments, this study revealed a considerable level of functional diversity and complexity, suggesting that dune plant species have evolved species-specific strategies to survive by partitioning growth-limiting resources.





Similar content being viewed by others
References
Abrams P (1983) The theory of limiting similarity. Annu Rev Ecol Syst 14:359–376
Ackerly DD (2004) Adaptation, niche conservatism, and convergence: comparative studies of leaf evolution in the California chaparral. Am Nat 163:654–671
Adler PB, Fajardo A, Kleinhesselink AR, Kraft NJB (2013) Trait-based tests of coexistence mechanisms. Ecol Lett 16:1294–1306
Andersen KM, Endara MJ, Turner BL, Dallin JW (2012) Trait-based community assembly of understory palms along a soil nutrient gradient in a lower montane tropical forest. Oecologia 168:519–531
Angert AL, Huxman TE, Chesson P, Venable DL (2009) Functional tradeoffs determine species coexistence via the storage effect. PNAS 106:11641–11645
Armstrong RA, McGehee R (1980) Competitive exclusion. Am Nat 115:151–170
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Bermúdez R, Retuerto R (2013) Living the difference: alternative functional designs in five perennial herbs coexisting in a coastal dune environment. Funct Plant Biol 40:1187–1198
Chesson P (1994) Multispecies competition in variable environments. Theor Popul Biol 45:227–276
Chesson P (2000) Mechanims of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Chesson P, Gebauer RLE, Schwinning S, Huntly N, Wiegand K, Ernest MSK, Sher A, Novoplansky A, Weltzin JF (2004) Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 141:236–253
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380
Crawford RMM (2008) Plants at the margin: ecological limits and climate change. Cambridge University Press, Cambridge
Culver DC (1970) Analysis of simple cave communities: niche separation and species packing. Ecology 51:949–958
Daniela C, Forino LMC, Balestri M, Pagni A (2009) Leaf anatomical adaptations of Calystegia soldanella, Euphorbia paralias and Otanthus maritimus to the ecological conditions of coastal sand dune systems. Caryologia 62:142–151
Díaz S, Cabido M (2001) Vive la difference: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Dijkstra P (1989) Cause and effect of differences in specific leaf area. In: Lambers H, Cambridge ML, Connings H, Pons TL (eds) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic, the Hague, pp 125–141
Dunham RJ, Nye PH (1976) The influence of soil water content on the uptake of ions by roots. III. Phosphate, potassium, calcium and magnesium uptake and concentration gradients in soil. J Appl Ecol 13:967–984
Elhaak MA, Migahid MM, Wegmann K (1997) Ecophysiological studies on Euphorbia paralias under soil salinity and sea water spray treatments. J Arid Environ 35:459–471
Evans J (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Filella I, Peñuelas J (2003) Partitioning of water and nitrogen in co-occurring Mediterranean woody shrub species of different evolutionary history. Oecologia 137:51–61
Fowler N (1988) The effects of environmental heterogeneity in space and time on the regulation of populations and communities. In: Davy AJ, Hutchings MJ (eds) Plant population ecology. Blackwell, Oxford, pp 249–269
Gendron RP (1987) Models and mechanisms of frequency-dependent predation. Am Nat 130:603–623
Gouvra E, Grammatikopoulos G (2003) Beneficial effects of direct foliar water uptake on shoot water potential of five chasmophytes. Can J Bot 81:1280–1286
Griffiths H (1992) Carbon isotope discrimination and the integration of carbon assimilation pathways in terrestrial CAM plants: commissioned review. Plant Cell Environ 15:1051–1062
Grime JP (2006) Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. J Veg Sci 17:255–260
Gutschick VP (1981) Evolved strategies in nitrogen acquisition by plants. Am Nat 118:607–637
Hastings A (1980) Disturbance, coexistence, history, and competition for space. Theor Popul Biol 18:363–373
Hesp PA (1991) Ecological processes and plant adaptations on coastal dunes. J Arid Environ 21:165–191
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901–908
Houlton BZ, Sigman DM, Schuur EAG, Hedin LO (2007) A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proc Natl Acad Sci USA 104:8902–8906
Intergovernmental Panel on Climate Change (2004) Contribution of working groups I, II and III to the fourth assessment report of the Intergovernmental Panel on Climate Change. Geneva
Kahmen A, Wanek W, Buchmann N (2008) Foliar delta(15)N values characterize soil N cycling and reflect nitrate or ammonium preference of plants along a temperate grassland gradient. Oecologia 156:861–870
Karlsson PS (1994) Photosynthetic capacity and photosynthetic Nutrient-Use Efficiency of Rhododendron lapponicum leaves as related to leaf nutrient status, leaf age and branch reproductive status. Funct Ecol 8:694–700
Knops JMH, Reinhart K (2000) Specific leaf area along a nitrogen fertilization gradient. Am Midl Nat 144:265–272
Korner C, Farquhar GD, Roksandic Z (1988) A global survey of carbon isotope discrimination in plants from high altitude. Oecologia 74:623–632
Lechowicz MJ, Bell G (1991) The ecology and genetics of fitness in forest plants. II. Microspatial heterogeneity of the edaphic environment. J Ecol 79:687–696
Lo Gullo MA, Salleo S (1988) Different strategies of drought resistance in three Mediterranean sclerophyllous trees growing in the same environmental conditions. New Phytol 108:267–276
Loik ME, Still CJ, Huxman TE, Harte J (2004) In situ photosynthetic freezing tolerance for plants exposed to a global warming manipulation in the Rocky Mountains, Colorado, USA. New Phytol 162:331–341
Ludlow MM (1989) Strategies of response to water stress. In: Kreeb KH, Richter H, Hinckley M (eds) Structural and functional responses to environmental stresses. SPB Academic Publishing, The Netherlands, pp 269–281
Ma JY, Sun W, Sun HL, Wang SM (2012) Stable carbon isotope characteristics of desert plants in the Junggar Basin, China. Ecol Res 27:115–124
Maun MA (2009) The biology of coastal sand dunes. Oxford University Press, New York
McKane RB, Grigal DF, Russelle MP (1990) Spatiotemporal differences in 15N uptake and the organization of an old-field plant community. Ecology 71:1126–1183
McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Giblin AE, Kielland K, Kwiatkowski BL, Laundre JA, Murray G (2002) Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415:68–71
Meinzer FC (2003) Functional convergence in plant responses to the environment. Oecologia 134:1–11
Miller AE, Bowman WD (2002) Variation in nitrogen-15 natural abundance and nitrogen uptake traits among co-occurring alpine species: do species partition by nitrogen form? Oecologia 130:609–616
Mouillot D, Mason NWH, Wilson JB (2007) Is the abundance of species determined by their functional traits? A new method with a test using plant communities. Oecologia 152:729–737
Nadelhoffer K, Shaver G, Fry B, Giblin A, Johnson L, McKane R (1996) 15N natural abundances and N use by tundra plants. Oecologia 107:386–394
Nouvellon Y, Laclau JP, Epron D, Kinana A, Mabiala A, Roupsard O, Bonnefond J-M, le Maire G, Marsden C, Bontemps J-D, Saint-André L (2010) Within-stand and seasonal variations of specific leaf area in a clonal Eucalyptus plantation in the Republic of Congo. For Ecol Manage 259:1796–1807
Olsen SR, Cole CU, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circ 939:1–19
Orlanski I (1975) A rational subdivision of scales for atmospheric processes. Bull Amer Meteorol Soc 56:527–530
Peñuelas J, Munné-Bosch S, Llusia J, Filella I (2004) Leaf reflectance and photo- and antioxidant protection in field-grown summer-stressed Phillyrea angustifolia. Optical signals of oxidative stress? New Phytol 162:115–124
Poorter H, van der Werf AK (1998) Is inherent variation in RGR determined by LAR at low irradiance and NAR at high irradiance? A review of herbaceous species. In: Lambers H, Poorter H, van Vuuren M (eds) Inherent variation in plant growth: Physiological mechanisms and ecological consequences. Backhuys, Leiden, pp 309–336
R Development Core Team (2009) R: A language and environment for statistical computing. http://www.R-project.org
Rasband WS (1997) ImageJ. http://imagej.nih.gov/ij/
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Reich PB, Buschena C, Tjoelker MG, Wrage K, Knops J, Tilman D, Machado JL (2003) Variation in growth rate and ecophysiology among 34 grassland and savanna species under contrasting N supply: a test of functional group differences. New Phytol 157:617–631
Retuerto R, Woodward FI (1992) Effects of windspeed on the growth and biomass allocation of white mustard Sinapis alba L. Oecologia 92:113–123
Retuerto R, Woodward FI (1993) The influences of increased CO2 and water-supply on growth, biomass allocation and water-use efficiency of Sinapis alba L grown under different wind speeds. Oecologia 94:415–427
Retuerto R, Fernández Lema B, Rodríguez Roiloa S, Obeso JR (2000) Gender, light and water effects in carbon isotope discrimination, and growth rates in the dioecious tree Ilex aquifolium. Funct Ecol 14:529–537
Robinson JV, Sandgren CD (1983) The effect of temporal environmental heterogeneity on community structure: a replicated experimental study. Oecologia 57:98–102
Robinson D, Handley LL, Scrimgeour CM, Gordon DC, Forster BP, Ellis RP (2000) Using stable isotope natural abundances (delta N-15 and delta C-13) to integrate the stress responses of wild barley (Hordeum spontaneum C. Koch.) genotypes. J Exp Bot 51:41–50
Rubio-Casal AE, Leira-Doce P, Figueroa ME, Castillo JM (2010) Contrasted tolerance to low and high temperatures of three tree taxa co-occurring on coastal dune forests under Mediterranean climate. J Arid Environ 74:429–439
Rundel PW, Esler KJ, Cowling RM (1999) Ecological and phylogenetic patterns of carbon isotope discrimination in the winter-rainfall flora of the Richtersveld, South Africa. Plant Ecol 142:133–148
Sala A, Sabaté S, Gracia C, Tenhunen JD (1994) Canopy structure within a Quercus ilex forested watershed: variations due to location, phenological development, and water availability. Trees 8:254–261
Salzman AG (1985) Habitat selection in a clonal plant. Science 228:603–604
Sandquist DR, Cordell S (2007) Functional diversity of carbon-gain, wateruse, and leaf-allocation traits in trees of a threatened lowland dry forest in Hawaii. Am J Bot 94:1459–1469
Sendall KM, Vourlitis GL, Lobo FA (2009) Seasonal variation in the maximum rate of leaf gas exchange of canopy and understory tree species in an Amazonian semi-deciduous forest. Braz J Plant Physiol 21:65–74
Sharifi MR, Meinzer FC, Nilsen ET, Rundel PW, Virginia RA, Jarrell WM, Herman DJ, Clark PC (1988) Effect of manipulation of water and nitrogen supplies on the quantitative phenology of Larrea tridentata (creosote bush) in the Sonoran desert of California. Am J Bot 75:1163–1174
Smedley MP, Dawson TE, Comstock JP, Donovan LA, Sherrill DE, Cook CS, Ehleringer JR (1991) Seasonal carbon isotope discrimination in a grassland community. Oecologia 85:314–320
Stubbs WJ, Wilson JB (2004) Evidence for limiting similarity in a sand dune community. J Ecol 92:557–567
Tilman D (1987) Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecol Monogr 57:189–214
Tilman D (1988) Plant strategies and the dynamics and structure of plant communities. Princeton University Press, Princeton
van Arendonk JJCM, Niemann GJ, Boon JJ, Lambers H (1997) Effects of nitrogen supply on the anatomy and chemical composition of leaves of four grass species belonging to the genus Poa, as determined by image-processing analysis and pyrolysis mass spectrometry. Plant Cell Environ 20:881–897
van de Water PK, Leavitt SW, Betancourt JL (1994) Trends in stomatal density and 13C/12C ratios of Pinus flexilis needles during last glacial-interglacial cycle. Science 264:239–243
van der Werf A, van Nuenen M, Visser AJ, Lambers H (1993) Effects of N-supply on the rates of photosynthesis and shoot and root respiration of inherently fast- and slow-growing monocotyledonous species. Physiol Plant 89:563–569
Westoby M (1998) A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199:213–227
Westoby M (1999) Generalization in functional plant ecology: the species sampling problem, plant ecology strategy schemes, and phylogeny. In: Pugnaire FI, Valladares F (eds) Handbook of functional ecology. Dekker, New York, pp 847–872
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Xu ZZ, Zhou GS (2008) Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J Exp Bot 59:3317–3325
Zar JH (1984) Biostatistical analysis. Pertinence-Hall International, New York
Acknowledgments
We greatly appreciate comments from two anonymous reviewers and the exhaustive revision and suggestions made by the handling editor Jeremy Lichstein that improved earlier versions of the manuscript. We thank to Felipe Macias (Department of Edaphology, USC) for soil analyses. R. Bermúdez was supported by a grant from the MEC (FPU, pre-doctoral training programme).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jeremy Lichstein.
R. Bermúdez and R. Retuerto contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bermúdez, R., Retuerto, R. Together but different: co-occurring dune plant species differ in their water- and nitrogen-use strategies. Oecologia 174, 651–663 (2014). https://doi.org/10.1007/s00442-013-2820-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2820-7