Abstract
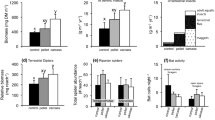
Reciprocal subsidies occur when ecosystems are paired, both importing and exporting resources to each other. The input of subsidies increases reciprocal subsidy export, but it is unclear how this changes with other important factors, such as ambient resources. We provide a conceptual framework for reciprocal subsidies and empirical data testing this framework using a pond–forest system in Missouri, USA. Our experiment used in situ pond mesocosms and three species of anurans: wood frogs, American toads, and southern leopard frogs. We predicted that increases in ambient resources (primary productivity) and detrital subsidy input (deciduous tree leaves) into pond mesocosms would increase reciprocal export (frog biomass) to the surrounding terrestrial ecosystem. In contrast, we found that increases in primary productivity consistently decreased frog biomass, except with leaf litter inputs. With leaf inputs, primary productivity did not affect the export of frogs, indicating that leaf detritus and associated microbial communities may be more important than algae for frog production. We found that subsidy inputs tended to increase reciprocal exports, and thus partial concordance with our conceptual framework.



Similar content being viewed by others
References
Alford RA (1999) Ecology: resource use, competition, and predation. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. The University of Chicago Press, Chicago, pp 240–278
Altig R, Whiles MR, Taylor CL (2007) What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshw Biol 52:386–395
Alvarez D, Nicieza AG (2002) Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct Ecol 16:640–648
Baxter CV, Fausch KD, Murakami M, Chapman PL (2004) Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 85:2656–2663
Baxter CV, Fausch KD, Saunders WC (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw Biol 50:201–220
Biggs BJ (1996) Patterns in benthic algae of streams. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Elsevier, San Diego, pp 31–56
Boone MD, Semlitsch RD, Little EE, Doyle MC (2007) Multiple stressors in amphibian communities: effects of chemical contamination, bullfrogs, and fish. Ecol Appl 17:291–301
Bridges CM (2000) Long-term effects of pesticide exposure at various life stages of the Southern Leopard Frog (Rana sphenocephala). Arch Environ Contam Toxicol 39:91–96
Diaz-Paniagua C (1985) Larval diets related to morphological characters of five anuran species in the biological reserve of Doñana (Huelva, Spain). Amphib Reptilia 6:307–321
Diaz-Paniagua C (1989) Larval diets of two anuran species, Pelodytes punctatus and Bufo bufo, in SW Spain. Amphib Reptilia 10:71–75
Earl JE, Luhring TM, Williams BK, Semlitsch RD (2011) Biomass export of salamanders and anurans from ponds is affected differentially by changes in canopy cover. Freshw Biol 56:2473–2482
Fontaine TD III, Ewel KC (1981) Metabolism of a Florida lake ecosystem. Limnol Oceanogr 26:754–763
Gee JHR, Smith BD, Lee KM, Griffiths SW (1997) The ecological basis of freshwater pond management for biodiversity. Aquat Conserv 7:91–104
Gibbons JW, Winne CT, Scott DE, Willson JD, Glaudas X, Andrews KM, Todd BD, Fedewa LA, Wilkinson L, Tsaliagos RN, Harper SJ, Greene JL, Tuberville TD, Metts BS, Dorcas ME, Nestor JP, Young CA, Arkre T, Reed RN, Buhlmann KA, Norman J, Croshaw DA, Hagen C, Rothermel BB (2006) Remarkable amphibian biomass and abundance in an isolated wetland: implications for wetland conservation. Conserv Biol 20:1457–1465
Gosner KL (1960) A simple table for staging anuran embryos with notes on identification. Herpetologica 16:183–190
Greig HS, Kratina P, Thompson PL, Palen WJ, Richardson JS, Shurin JB (2012) Warming, eutrophication, and predator loss amplify subsidies between aquatic and terrestrial ecosystems. Glob Change Biol 18:504–514
Harkey GA, Semlitsch RD (1988) Effects of temperature on growth, development, and color polymorphism in the ornate chorus frog Pseudacris ornata. Copeia 1988:1001–1007
Harper EB, Semlitsch RD (2007) Density dependence in the terrestrial life history stage of two anurans. Oecologia 153:879–889
Hocking DJ, Semlitsch RD (2007) Effects of timber harvest on breeding-site selection by gray treefrogs (Hyla versicolor). Biol Conserv 138:506–513
Hocking DJ, Semlitsch RD (2008) Effects of experimental clearcut logging on gray treefrog (Hyla versicolor) tadpole performance. J Herpetol 42:689–698
Huxel GR, Polis GA, Holt RD (2004) At the frontier of the integration of food web ecology and landscape ecology. In: Polis GA, Power M, Huxel GR (eds) Food webs at the landscape level. University of Chicago Press, Chicago, pp 434–451
Janetski DJ, Chaloner DT, Tiegs SD, Lamberti GA (2009) Pacific salmon effects on stream ecosystems: a quantitative synthesis. Oecologia 159:583–595
Kraus JM, Pletcher LT, Vonesh JR (2011) Variation in active and passive resource inputs to experimental pools: mechanisms and possible consequences for food webs. Freshw Biol 56:491–502
Lanoo M (2005) Amphibian declines: the conservation status of United States species. University of California Press, Berkeley
Lay JP, Peither A, Jüttner I, Weiss K (1993) In situ pond mesocosms for ecotoxicological long-term studies. Chemosphere 26:1137–1150
Leeper DA, Taylor BE (1998) Abundance, biomass and production of aquatic invertebrates in Rainbow Bay, a temporary wetland in South Carolina, USA. Arch Hydrobiol 143:335–362
Leroux SJ, Loreau M (2008) Subsidy hypothesis and strength of trophic cascades across ecosystems. Ecol Lett 11:1147–1156
Loman J (2001) Effects of tadpole grazing on periphytic algae in ponds. Wetl Ecol Manag 9:135–139
Loreau M, Holt RD (2004) Spatial flows and the regulation of ecosystems. Am Nat 163:606–615
Marcarelli AM, Baxter CV, Mineau MM, Hall RO Jr (2011) Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92:1215–1225
Marczak LB, Thompson RM, Richardson JS (2007) Meta-analysis: trophic level, habitat, and productivity shape the food web effects of resource subsidies. Ecology 88:140–148
Mitchell RJ (2001) Path analysis: pollination. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments, 2nd edn. Oxford University Press, Oxford
Mokany A, Wood JT, Cunningham SA (2008) Effect of shade and shading history on species abundances and ecosystem processes in temporary ponds. Freshw Biol 53:1917–1928
Murakami M, Nakano S (2002) Indirect effect of aquatic insect emergence on a terrestrial insect population through by birds predation. Ecol Lett 5:333–337
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA 98:166–170
Ostrofsky ML (1997) Relationship between chemical characteristics of autumn-shed leaves and aquatic processing rates. J North Am Benthol Soc 16:750–759
Persson L, Bengtsson J, Menge BA, Power M (1996) Productivity and consumer regulation: concepts, patterns and mechanisms. In: Polis GA, Winemiller KO (eds) Food webs: integration of patterns and dynamics. Chapman and Hall, New York
Peterman WE, Crawford JA, Semlitsch RD (2008) Productivity and significance of headwater streams: Population structure and biomass of the black-bellied salamander (Desmognathus quadramaculatus). Freshw Biol 53:347–357
Polis GA, Strong DR (1996) Food web complexity an community dynamics. Am Nat 147:813–846
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316
Potvin C (2001) ANOVA: experimental layout and analysis. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, New York
Quinn TP, Carlson SM, Gende SM, Rich HB Jr (2009) Transportation of Pacific salmon carcasses from streams to riparian forests by bears. Can J Zool 87:195–203
Regester KJ, Lips KR, Whiles MR (2006) Energy flow and subsidies associated with the complex life cycle of ambystomatid salamanders in ponds and adjacent forest in southern Illinois. Oecologia 147:303–314
Rubbo MJ, Kiesecker JM (2004) Leaf litter composition and community structure: Translating regional species changes into local dynamics. Ecology 85:2519–2525
Rubbo MJ, Belden LK, Kiesecker JM (2008) Differential responses of aquatic consumers to variations in leaf-litter inputs. Hydrobiologia 605:37–44
SAS (2004) SAS/STAT user’s guide. SAS Institute, Cary
Schiesari L (2006) Pond canopy cover: a resource gradient for anuran larvae. Freshw Biol 51:412–423
Schiesari L, Werner EE, Kling GW (2009) Carnivory and resource-based niche differentiation in anuran larvae: implications for food web and experimental ecology. Freshw Biol 54:572–586
Schulman RS, Chase JM (2007) Increasing isolation reduces predator:prey species richness ratios in aquatic food webs. Oikos 116:1581–1587
Semlitsch RD, Scott DE, Pechman JHK, Gibbons JW (1996) Structure and dynamics of an amphibian community. In: Cody ML, Smallwood JA (eds) Long-term studies of vertebrate communities. Academic, San Diego, pp 217–248
Semlitsch RD, Conner CA, Hocking DJ, Rittenhouse TAG, Harper EB (2008) Effects of timber harvesting on pond-breeding amphibian persistence: testing the evacuation hypothesis. Ecol Appl 18:283–289
Semlitsch RD, Boone MD (2009) Aquatic mesocosms. In: Dodd CK Jr (ed) Ecology and conservation of amphibians: a handbook of techniques. Oxford University Press, Oxford
Semlitsch RD, Todd BD, Blomquist SM, Calhoun AJK, Gibbons JW, Gibbs JP, Graeter GJ, Harper EB, Hocking DJ, Hunter ML Jr, Patrick DA, Rittenhouse TAG, Rothermel BB (2009) Effects of timber harvest on amphibian populations: understanding mechanisms from forest experiments. Bioscience 59:853–862
Skelly DK, Freidenburg LK, Kiesecker JM (2002) Forest canopy and the performance of larval amphibians. Ecology 83:983–992
Smith-Gill SJ, Berven KA (1979) Predicting amphibian metamorphosis. Am Nat 113:563–585
Søndergaard M, Jeppsesen E, Jensen JP (2005) Pond or lake: does it make any difference? Arch Hydrobiol 162:143–165
Travis J (1980) Phenotypic variation and the outcome of interspecific competition in hylid tadpoles. Evolution 34:40–50
Tylianakis JM, Didham RK, Wratten SD (2004) Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 85:658–666
USDA, NRCS (2010) The PLANTS database. National Plant Data Center, Baton Rouge. http://plants.usda.gov
Wallace JB, Eggert SL, Meyer JL, Webster JR (1997) Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277:102–104
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Wetzel RG, Likens GE (2000) Limnological analysis, 3rd edn. Springer, New York
Whiles MR, Goldowitz BS (2001) Hydrologic influences on insect emergence production from central Platte River wetlands. Ecol Appl 11:1829–1842
Whiles MR, Gladyshev MI, Sushchik NN, Makhutova N, Kalachova GS, Peterson SD, Regester KJ (2010) Fatty acid analyses reveal high degrees of omnivory and dietary plasticity in pond-dwelling tadpoles. Freshw Biol 55:1533–1547
Williams BK, Rittenhouse TAG, Semlitsch RD (2008) Leaf litter input mediates tadpole performance across forest canopy treatments. Oecologia 155:377–384
Acknowledgments
We thank K. Cohagen and P. Castello for help in the field, J. Fairchild for helpful advice, P. Castello, D. Drake, S. Olson, and L. Johnson for help in the laboratory, and C. Rabeni, R. Holdo, J. Chase, C. Shulse, and several anonymous reviewers for helpful comments on previous versions of this manuscript. Financial support was provided by the National Science Foundation (DEB-0239943) and the MU Alumni Association. JEE was supported by a Life Sciences Fellowship, a TWA Scholarship, and a Conservation Biology Fellowship through the University of Missouri, as well as an Environmental Protection Agency STAR Fellowship. Research was conducted with Missouri Department of Conservation Wildlife Collecting Permits 13759, 14119, and 14467 and under University of Missouri Animal Care Protocols 3368 and 6144.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ross Alford.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Earl, J.E., Semlitsch, R.D. Reciprocal subsidies in ponds: does leaf input increase frog biomass export?. Oecologia 170, 1077–1087 (2012). https://doi.org/10.1007/s00442-012-2361-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2361-5