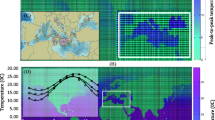
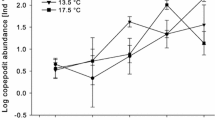
Abstract
A major goal of modern ecology is to understand macroecological patterns based on their mechanistic underpinnings. The metabolic theory of ecology predicts a monotonic increase of biodiversity with temperature based on the principles of metabolism. For marine copepods, observations have shown that while biodiversity does increase with temperature, the theory’s prediction overestimates the slope of this relationship by a factor of two. By relaxing the theory’s assumption that size is invariant with respect to temperature, and by incorporating a mechanistic description of copepod development into the theory, we provide an adjusted prediction that agrees with the observed relationship. The addition of development into the theory adds the potential to refine the prediction for a wider range of taxa, to account for discrepancies between prediction and observations, and to describe a wider variety of temperature–richness relationships.


Similar content being viewed by others
References
Allen AP, Brown JH, Gillooly JF (2002) Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297:1545–1548
Arrhenius S (1889) Über die reaktionsgeschwindigkeit bei der inversion von rohrzucker durch sauren. Z Phys Chem 4(7):226–248
Brown JH, Sibly RM (2006) Life-history evolution under a production constraint. Proc Natl Acad Sci USA 103(47):17595–17599
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85(7):1771–1789
B\(\breve{\hbox{e}}\)lehrádek J (1935) Temperature and living matter. Protoplasma Monog 8:1–277
Campbell RG, Wagner MM, Teegarden GJ, Boudreau CA, Durbin EG (2001) Growth and development rates of the copepod Calanus finmarchicus reared in the laboratory. Mar Ecol Prog Ser 221:161–183
Currie DJ, Mittelbach GG, Cornell HV, Field R, Gugan JF, Hawkins BA, Kaufman DM, Kerr JT, Oberdorff T, O’Brien E, Turner JRG (2004) Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol Lett 7(12):1121–1134
Deevey GB (1960) Relative effects of temperature and food on seasonal variations in length of marine copepods in some eastern American and western European waters. Bull Bingham Oceanogr Collect 17(2):54–86
Forster J, Hirst AG, Woodward G (2011) Growth and development rates have different thermal responses. Am Nat 178(5):668–678
Frost BW (1980) The inadequacy of body size as an indicator of niches in the zooplankton. In: Kerfoot WC (ed) Evolution and ecology of zooplankton communities. University Press of New England, Hanover, pp 742–753
Glazier DS (2010) A unifying explanation for diverse metabolic scaling in animals and plants. Biol Rev 85(1):111–138
Halsband-Lenk C, Hirche HJ, Carlotti F (2002) Temperature impact on reproduction and development of congener copepod populations. J Exp Mar Biol Ecol 271(6):121–153
van der Have T, de Jong G (1996) Adult size in ectotherms: temperature effects on growth and differentiation. J Theor Biol 183(3):329–340
Hawkins BA, Albuquerque FS, Arajo MB, Beck J, Bini LM, Cabrero-Sanudo FJ, Castro-Parga I, Diniz-Filho JAF, Ferrer-Castn D, Field R, Gmez JF, Hortal J, Kerr JT, Kitching IJ, Len-Corts JL, Lobo JM, Montoya D, Moreno JC, Olalla-Trraga M, Pausas JG, Qian H, Rahbek C, Rodrguez M, Sanders NJ, Williams P (2007) A global evaluation of metabolic theory as an explanation for terrestrial species richness gradients. Ecology 88(8):1877–1888
Heip C, Smol N (1976) Influence of temperature on the reproductive potential of two brackish-water harpacticoids (Crustacea: Copepoda). Mar Biol 35:327–334
Huntley ME, Lopez MDG (1992) Temperature-dependent production of marine copepods: a global synthesis. Am Nat 140(2):201–242
Kiørboe T (2008) A mechanistic approach to plankton ecology. Princeton University Press, Princeton
Lee HW, Ban S, Ikeda T, Matsuishi T (2003) Effect of temperature on development, growth and reproduction in the marine copepod Pseudocalanus newmani at satiating food condition. J Plankton Res 25(3):261–271
Li BL, Gorshkov VG, Makarieva AM (2004) Energy partitioning between different-sized organisms and ecosystem stability. Ecology 85(7):1811–1813
Liang D, Uye S, Onb T (1996) Population dynamics and production of the planktonic copepods in a eutrophic inlet of the inland sea of japan. I. Centropages abdominalis. Mar Biol 124:527–536
Maps F, Pershing AJ, Record NR (2011) A generalized approach for simulating growth and development in diverse marine copepod species. ICES J Mar Sci. doi:10.1093/icesjms/fsr182
Mauchline J (1998) The biology of calanoid copepods. Adv Mar Biol 33:1–710
McLaren IA (1966) Adaptive significance of large size and long life of the chaetognath Sagitta elegans in the arctic. Ecology 47(5):852–855
McLaren IA, Corkett CJ, Zillioux EJ (1969) Temperature adaptations of copepod eggs from the arctic to the tropics. Biol Bull 137:486–493
van der Meer J (2006) Metabolic theories in ecology. Trends Ecol Evol 21(3):136–140
Padmavati G, Ikeda T (2002) Development of Metridia pacifica (Crustacea: Copepoda) reared at different temperatures in the laboratory. Plankton Biol Ecol 49:93–96
Peterson WT (1986) Development, growth, and survivorship of the copepod Calanus marshallae in the laboratory. Mar Ecol Prog Ser 29:61–72
Razouls C (1974) Variations annuelles quantitatives de deux especes dominantes de copepodes planctoniques Centropages typicus et Temora stylifera de la region de banyuls: cycles biologiques et estimations de la production. III. dynamique des populations et calcul dev. Cah Biol Mar 15:51–88
Rombouts I, Beaugrand G, Ibañez F, Chiba S, Legendre L (2011) Marine copepod diversity patterns and the metabolic theory of ecology. Oecologia 166(22–26):349–355
Savage V, Gillooly J, Brown J, West G, Charnov E (2004) Effects of body size and temperature on population growth. Am Nat 163(3):429–441
Thompson BM (1982) Growth and development of Pseudocalanus elongatus and Calanus sp. in the laboratory. J Mar Biol Assoc UK 62:359–372
Uye SI (1980) Development of neritic copepods Acartia clausi and A. steueri. Bull Plankton Soc Japan 27(1):11–18
Uye SI (1988) Temperature-dependent development and growth of Calanus sinicus (Copepoda: Calanoida) in the laboratory. Hydrobiologia 167–168(1):285–293
Vidal J (1980) Physioecology of zooplankton. II. Effects of phytoplankton concentration, temperature, and body size on the development and molting rates of Calanus pacificus and Pseudocalanus sp. Mar Biol 56(2):135–146
Zimmerman KM, Pechenik JA (1991) How do temperature and salinity affect relative rates of growth, morphological differentiation, and time to metamorphic competence in larvae of the marine gastropod Crepidula plana? Biol Bull 180:372–386
Zuo W, Moses ME, West GB, Hou C, Brown JH (2011) A general model for effects of temperature on ectotherm ontogenetic growth and development. Proc R Soc Lond B
Acknowledgments
Support from the National Science Foundation’s Biological Oceanography program (OCE-0962074). Thanks to Jeff Runge for valuable input. Thanks to anonymous reviewers for contributing some very insightful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marc Mangel.
Rights and permissions
About this article
Cite this article
Record, N.R., Pershing, A.J. & Maps, F. First principles of copepod development help explain global marine diversity patterns. Oecologia 170, 289–295 (2012). https://doi.org/10.1007/s00442-012-2313-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2313-0