Abstract
The immune system is an important player in individual trade-offs, but what has rarely been explored is how different strategies of investment in immune response may affect reproductive decisions. We examined the relationship between the strength of maternal immune response and offspring viability and immune response in captive zebra finches Taeniopygia guttata. In three independent experiments, the females and subsequently their adult offspring were challenged with sheep red blood cells, and their responses were measured. There was no relationship between offspring immune response and that of their mothers. However, we found offspring survival until adulthood to be negatively related to maternal antibody titers. That effect was consistent among all experiments and apparent despite the fact that we partially cross-fostered newly hatched nestlings between nests of different females. This suggests that the observed effects of maternal immune response is not mediated by potentially altered female rearing abilities. To our knowledge, this is the first study showing the relationship between the strength of the immune response and between-generational fitness costs in birds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life-history evolution is governed by trade-offs (Stearns 1992) which arise due to limited resources, time constraints, antagonistic effects of substances mediating different functions of the organism (e.g., Adamo et al. 2008) and/or negative genetic correlations (Sinervo and Svensson 1998; Sgro and Hoffmann 2004; McKean et al. 2008). Because the immune system has a strong impact on individual fitness, immunocompetence appears to provide an adequate framework to study evolutionary compromises, both at the phenotypic (see Viney et al. 2005; Zuk and Stoehr 2002; Knowles et al. 2009 for review) and genetic levels (e.g., Sgro and Hoffmann 2004; Cotter et al. 2008; McKean et al. 2008).
Extensive research have focused on intra-individual trade-offs involving immunity and reproductive performance, and birds are a popular model to study these issues (Viney et al. 2005; Zuk and Stoehr 2002; Knowles et al. 2009). The majority of studies of the potential effects of maternal immunization on offspring performance have concentrated on the early stages of offspring development. They provide evidence that immune challenge of the parents affects their rearing abilities and offspring performance. Some studies have shown that maternal immunization enhances offspring growth, suggesting that this effect was mediated via maternal agents transmitted in the eggs (e.g., Buechler et al. 2002; Lozano and Ydenberg 2002; Martyka et al. 2011). Furthermore, offspring exposed to the same antigen as that of their mothers grew faster compared to the offspring exposed to a novel antigen (Grindstaff 2008). The observed effects of immunization on the offspring performance might also be mediated by altered behavior of immunized parents, as demonstrated by decreased nest attendance and its consequences for growth and survival (Råberg et al. 2000; Ilmonen et al. 2000). We are aware of only two studies reporting long-term effects of maternal immunization in birds. Specifically, maternal immunization has been shown to alter immune response of the offspring produced the following year after immunization. The immune response of offspring produced a year after vaccinating a female was larger than that of offspring of non-vaccinated mothers in the song sparrow Melospiza melodia (Reid et al. 2006), while in the kittiwake Rissa tridactyla this effect was opposite (Staszewski et al. 2007). However, except for Reid et al.’s (2006) experiment, there are no studies which follow the effects of maternal immunization until offspring independence.
Here, we aim at investigating the relationship between the level of maternal immune response and offspring performance from egg until adulthood. If resources are limited, the mother investing heavily in self-maintenance (immune function) may devote fewer resources to reproduction and hence produce lower quality offspring or fewer of them. Thus, we predicted the negative relationship between maternal immune response and offspring performance. We used data from three independent studies performed on zebra finches Taeniopygia guttata in the same laboratory, which incorporated female inoculation with a novel antigen, sheep red blood cells (SRBCs). We explored whether maternal immune response influences offspring viability, i.e. hatching success and survival to maturity, as well as offspring immune response and body size at adulthood.
Materials and methods
General breeding procedures
In our laboratory colony, zebra finches are kept in air-conditioned chambers at 20 ± 2°C, under a 13:11-h incandescent light:dark photoperiod, lights on at 0700 hours. Birds are fed ad libitum with a standard mixture of seeds (Megan, Poland), along with a mixture of hard-boiled egg. Birds also receive a cuttlebone and grit. Rearing conditions are kept constant during breeding experiments. Initially, all birds are maintained in a common aviary, where they can mate freely and rear one brood. This aims at increasing their breeding experience. Sexes are then separated for few months and paired again in visually separated, individual cages (75 × 30 and 40 cm high) equipped with external nest boxes and nesting material. Following pairing, nest boxes are inspected every morning between 0900 and 1000 hours to record nest building and egg laying, as well as labeling new eggs with a non-toxic marker. At the day of expected hatching, nests are inspected hourly, whereas during nights (between 2000 and 0800 hours) eggs are transferred to separate compartments in an incubator chamber (humidity ~70%, temperature 36.4°C). This enables determination of which hatchling comes from which egg. Offspring that hatched in the incubator were returned to the nest at 0800 hours. At the age of 2 weeks, nestlings are ringed with individually numbered aluminium rings. Independent offspring are kept in a common aviary.
Subjects and immunization procedure
The dataset comprises 79 females that produced 389 offspring. Females belonged to three groups differing in the timing of maternal immunization in relation to the breeding stage and in the degree of hatching asynchrony of the offspring. Group 1 consisted of 41 females which were immunized 5 months before breeding. Group 2 consisted of 18 females paired with males 14 days after the first immunization. Those females were again injected with SRBCs on the day of laying the first egg. Group 3 consisted of 20 females that were treated in the same way as group 2 and, additionally, as a part of another experiment, had hatching of their offspring synchronized. In those clutches, newly laid eggs were removed from the nest within 3 h of laying and replaced by clay models. Removed eggs were stored at ca. 9°C and returned to the nest the day after the clutch was completed (see Rutkowska and Cichoń 2005 for details in the procedure). The three groups of females were breeding at different times, but in each case some of their offspring (overall 59%) were cross-fostered. Specifically, whenever possible, two or three chicks from each nest were cross-fostered within a pair of broods which started hatching on the same day and had similar clutch size (±1 egg). Hatchlings were cross-fostered at the day of hatching and were matched according to the position of the egg in the laying sequence. The cross-fostering procedure is very important as it allows testing of pre-hatching maternal effects while controlling of the potential effects of the post-hatching environment; offspring of the same mother are reared in different nests. Offspring survival was followed up to 3 months of age, when their immune response and body size were measured.
Females and their adult offspring were injected intra-abdominally with 0.1 ml of 40% suspension of SRBCs in phosphate buffer saline (PBS). Six days after injection, when antibody level reaches its highest level, ca. 100 μl of blood was taken from each bird for future assessment of primary antibody titers. A standard hemagglutination test was applied to quantify the specific antibody production against SRBCs (Hay and Hudson 1989). First, to remove the proteins that might lyse the cells, plasma samples were incubated at 56°C for 30 min. This procedure was followed by the dilution of each plasma sample serially in 10 μl of PBS in 96-well round-bottomed microtiter plates. After adding 10 μl of 10% suspension of SRBCs in PBS to such prepared dilutions and incubating the plates at 37°C for 1 h, the titers were scored. The antibody level was expressed as the log of the reciprocal of the lowest dilution showing agglutination. In all analyses, we used primary antibody titers.
Statistical analysis
In all analyses, the explanatory variables were female primary antibody titers introduced as a continuous predictor, group of females (independent experiments) introduced as a three-level fixed factor and the interaction between the two. The interaction was always removed if not significant (at P > 0.05). Since the three experiments differed in procedure and timing, the variable group was introduced only to account for differences between experiments but we avoid drawing any conclusions even if this variable appears significant. The cross-fostering procedure aimed at controlling potential effects of the post-hatching environment, but in order to further confirm that variation in offspring performance is indeed shaped by pre-hatching maternal effects regardless of potential post-hatching factors, we also ran models including both maternal and foster female antibody titers. The probability of hatching and survival of the offspring to the adulthood was modeled at the individual egg or offspring level with a generalized linear mixed model (GLIMMIX procedure in SAS) with a logit link function and binomial error variance (Krackow and Tkadlec 2001). To analyze variation in offspring immune response and body size, we used linear mixed model (PROC MIXED in SAS). Female identity and foster female identity were additionally included in all analyses as higher level random factors to account for relatedness between nestlings and the cross-fostering procedure. The statistical models employed here allow for controlling variation in random effects while testing significance of fixed effects, but do not allow formal testing of random effects. In the case of random effects, we report estimates of variance components and the significance of these estimates.
Results
Mean maternal immune response did not differ between the groups (F 2,76 = 0.48, P = 0.62) and it did not affect latency to clutch initiation after pairing (in all groups F 1,77 = 0.01, P = 0.91) or the clutch size (F 1,77 = 0.00, P = 0.96). Hatching success of the offspring was not affected by maternal antibody titers, group or their interaction (all P > 0.3). The probability of offspring survival to the adulthood was negatively affected by maternal antibody titers and it differed between the groups (Table 1). Mean probability of offspring survival (±95% CI) in Group 1 equalled 0.70 ± 0.06, in Group 2 0.88 ± 0.06 and in Group 3 0.82 ± 0.08. The interaction between maternal antibody titers and group (F 2,61.5 = 0.43, P = 0.65) was removed from the final model. Here, we also considered a model in which foster female antibody titers was additionally included. In this model, the effect of maternal antibody titers remained significant (F 1,55 = 6.22, P = 0.016), but the effect of foster female antibody titers appeared non-significant (F 1,212 = 0.00, P = 0.97). We did not find any significant relationship between maternal antibody titers and offspring immune response, body mass and tarsus length measured at adulthood (all P > 0.1). These offspring traits did not differ between the groups (all P > 0.1) except for immune function [F 2,118 = 8.32, P = 0.0004; mean (±95% CI), Group 1: 3.7 ± 0.4; Group 2: 3.2 ± 0.8; Group 3: 5.4 ± 0.7]. There were no interactions between maternal antibody titers and group. Models including foster female antibody titers showed that this variable had no effect on offspring immune response or body size.
Discussion
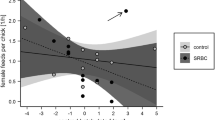
The immune function has repeatedly been shown to interact with life-history traits, including those related to reproduction (Warner et al. 1987; Råberg et al. 2000; Ilmonen et al. 2000), yet long-term consequences of parental immune response have rarely been addressed. Here, we show that offspring survival is negatively related to the magnitude of the female immune response (Table 1; Fig. 1). The observed relationship is consistent among three independent experiments and, more importantly, it is apparent despite the fact that we partially cross-fostered newly hatched nestlings between nests of different females. The latter suggests that the observed effects of maternal immune response is not mediated by potentially altered female rearing abilities.
Probability of survival until 3 months of the offspring of zebra finches Taeniopygia guttata in relation to immunocompetence of their mothers. Each point represents mean value for survival of the offspring of a given female. Point size indicates overlapping the same value of maternal primary antibody titer and offspring survival. Line is fitted as a logistic function of the binomial values (0 or 1) of individual offspring survival
The observed negative effect might result from reallocation of resources and decreased maternal condition related to mounting immune response. However, some females were immunized well before breeding and some while breeding, so the negative consequences of immunization should differ between the groups. The similar slopes observed in all three groups (lack of significant interaction between maternal antibody level and group and lack of the effect of foster female antibody level) suggest that the observed relationship is not likely to be mediated by the costs of mounting the immune response. Moreover, the costs of mounting immune response should have an immediate effect on the earliest stages of reproduction. Yet, latency to laying, clutch size and hatching success of the offspring were not related to maternal immune response. If any constraints related to immune response came into play during chick rearing, such effects would have been controlled for by the cross-fostering of some of the offspring. It is also implausible that offspring survival is mediated by some maternally transmitted agents like immunoglobulins or hormones deposited in the eggs. If so, one should expect the survival of offspring of immunized mothers to differ from that of non-immunized mothers. Average (±95% CI) survival observed in our population equals 0.80 ± 0.08, and the survival rates observed in this study range from 0.70 to 0.88 (±95% CI: 0.06–0.08). Thus, the direct effects of maternal immune challenge are not likely to explain variation in offspring survival.
Lower offspring survival of the mothers showing higher antibody production could be potentially mediated by maternal substances transmitted via the eggs that affect nestling survival. For example, maternal immunization has been shown to increase immunoglobulin (Hasselquist and Nilsson 2009) and to reduce carotenoid (Saino et al. 2002) and androgen (Gil et al. 2006) levels in the eggs. If the change in the concentration of these substances after immune challenge is proportional to the magnitude of the immune response, this could potentially explain differential offspring survival. However, the evidence for such subtle effects is so far lacking, and the above explanation seems unlikely. The observed negative relationship between maternal immune response and offspring survival may also reflect different life-history strategies. For instance, higher maternal immune function may manifest a strategy to favor investments in self maintenance, own condition and survival, which is likely to happen at the expense of investment into offspring generation (Cichoń 2001). Contrasting strategies of investment into immune function and other life-history traits have been reported in several animal taxa, such as birds (e.g., Råberg et al. 2000; Ilmonen et al. 2000; Gasparini et al. 2009), reptiles (Calsbeek et al. 2008) and insects (Cotter et al. 2008). The negative genetic correlation between resistance to pathogen and fecundity (number of emerged offspring) was documented in Drosophila (McKean et al. 2008) in the food-limited environment. In birds, there is some evidence for the negative genetic correlation between immunocomptence and egg size observed in the chickens selected for Marek’s disease (reviewed in Warner et al. 1987). To confirm the existence of contrasting allocation strategies into immune function and reproduction, future studies should measure female immune response after their breeding attempt.
We were not able to detect any relationship between maternal and offspring immune response, although immunocompetence seems to have a significant genetic component (Singel and Gross 1979; Kilpimaa et al. 2005). This may stem from potentially differential survival of offspring showing low and high immunocompetence that resulted in reduction of variation in immune response measured in adult offspring. It is also possible that offspring immune response could be shaped by synergistic or antagonistic effects of maternal antibodies and other compounds in the eggs (Hasselquist and Nilsson 2009), yet nature and mechanisms of such effects need future research.
References
Adamo SA, Roberts JL, Easy R, Ross NW (2008) Competition between immune function and lipid transport for the protein apolipophorin III leads to stress-induced immunosuppression in crickets. J Exp Biol 211:531–538. doi:10.1242/jeb.013136
Buechler K, Fitze PS, Gottstein B, Jacot A, Richner H (2002) Parasite-induced maternal response in a natural bird population. J Anim Ecol 71:247–252. doi:10.1046/j.1365-2656.2002.00591.x
Calsbeek R, Bonneaud C, Smith TB (2008) Differential fitness effects of immunocompetence and neighbourhood density in alternative female lizard morphs. J Anim Ecol 77:103–109. doi:10.1111/j.1365-2656.2007.01320.x
Cichoń M (2001) Diversity of age-specific reproductive rates may result from ageing and optimal resource allocation. J Evol Biol 14:180–185. doi:10.1046/j.1420-9101.2001.00243.x
Cotter SC, Myatt JP, Benskin CMH, Wilson K (2008) Selection for cuticular melanism reveals immune function and life-history trade-offs in Spodoptera littoralis. J Evol Biol 21:1744–1754. doi:10.1111/j.1420-9101.2008.01587.x
Gasparini J, Bize P, Piault R, Wakamatsu K, Blount JD, Ducrest A-L, Roulin A (2009) Strength and cost of an induced immune response are associated with a heritable melanin-based colour trait in female tawny owls. J Anim Ecol 78:608–616. doi:10.1111/j.1365-2656.2008.01521.x
Gil D, Marzal A, de Lope F, Puerta M, Møller AP (2006) Female house martins (Delichon urbica) reduce egg androgen deposition in response to a challenge of their immune system. Behav Ecol Sociobiol 60:96–100. doi:10.1007/s00265-005-0145-1
Grindstaff JL (2008) Maternal antibodies reduce costs of an immune response during development. J Exp Biol 211:654–660. doi:10.1242/jeb.012344
Hasselquist D, Nilsson JÅ (2009) Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Philos Trans R Soc Lond B 364:51–60. doi:10.1098/rstb.2008.0137
Hay L, Hudson FC (1989) Practical immunology. Blackwell, Oxford
Ilmonen P, Taarna T, Hasselquist D (2000) Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc R Soc Lond B 267:665–670. doi:10.1098/rspb.2000.1053
Kilpimaa J, Van De Casteele T, Jokinen I, Mappes J, Alatalo RV (2005) Genetic and environmental variation in antibody and T-cell mediated responses in the great tit. Evolution 59:2483–2489. doi:10.1554/04-678.1
Knowles SCL, Nakagawa S, Sheldon BC (2009) Elevated reproductive effort increases blood parasitemia levels and decreases immune function in birds: a meta-regression approach. Funct Ecol 23:405–415. doi:10.1111/j.1365-2435.2008.01507.x
Krackow S, Tkadlec E (2001) Analysis of brood sex ratios: implications of offspring clustering. Behav Ecol Sociobiol 50:293–301. doi:10.1007/s002650100366
Lozano GA, Ydenberg RC (2002) Transgenerational effects of maternal immune challenge in tree swallows (Tachycineta bicolor). Can J Zool 80:918–925. doi:10.1139/z02-063
Martyka R, Rutkowska J, Cichoń M (2011) Sex-specific effects of maternal immunization on yolk antibody transfer and offspring performance in zebra finches. Biol Lett 7:50–53. doi:10.1098/rsbl.2010.0549
McKean K, Yourth C, Lazzaro B, Clark A (2008) The evolutionary costs of immunological maintenance and deployment. BMC Evol Biol 8:76. doi:10.1186/1471-2148-8-76
Råberg L, Nilsson J-Å, Ilmonen P, Stjernman M, Hasselquist D (2000) The cost of an immune response: vaccination reduces parental effort. Ecol Lett 3:382–386. doi:10.1046/j.1461-0248.2000.00154.x
Reid JM, Arcese P, Keller LF, Hasselquist D (2006) Long term maternal effect on offspring immune response in song sparrows Melospiza melodia. Biol Lett 2:573–576. doi:10.1098/rsbl.2006.0544
Rutkowska J, Cichoń M (2005) Egg size, offspring sex and hatching asynchrony in zebra finches Taeniopygia guttata. J Avian Biol 36:12–17. doi:10.1111/j.0908-8857.2005.03469.x
Saino N, Bertacche V, Ferrari RP, Martinelli R, Møller AP, Stradi R (2002) Carotenoid concentration in barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proc R Soc Lond B 269:1729–1733. doi:10.1098/rspb.2002.2088
Sgro CM, Hoffmann AA (2004) Genetic correlations, tradeoffs and environmental variation. Heredity 93:241–248
Sinervo B, Svensson E (1998) Mechanistic and selective causes of life history trade-offs and plasticity. Oikos 83:432–442
Singel PB, Gross WB (1979) Production and persistence to antibodies in chickens to sheep erythrocytes. 1. Directional selection. Poultry Sci 59:1–5
Staszewski V, Gasparini J, Mccoy KD, Tveraa T, Boulinier T (2007) Evidence of an interannual effect of maternal immunization on the immune response of juveniles in a long-lived colonial bird. J Anim Ecol 76:1215–1223. doi:10.1111/j.1365-2656.2007.01293.x
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Viney ME, Riley EM, Buchanan KL (2005) Optimal immune responses: immunocompetence revisited. Trends Ecol Evol 20:665–669. doi:10.1016/j.tree.2005.10.003
Warner CM, Meeker DL, Rothschild MF (1987) Genetic control of immune responsiveness: a review of its use as a tool for selection for disease resistance. J Anim Sci 64:394–406
Zuk M, Stoehr AM (2002) Immune defense and host life history. Am Nat 160:S9–S22. doi:10.1086/342131
Acknowledgments
We thank E. Podmokła, K. Kot and A. Solecka for help with taking care of the birds and S. Drobniak for statistical advice. The study was supported by the Ministry of Science and Higher Education in years 2006–2010 (grants N30405331/2019, N30400931/0397), and partly by DS/WBiNoZ/INoŚ/757/07-09.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Heli Siitari.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Rutkowska, J., Martyka, R., Arct, A. et al. Offspring survival is negatively related to maternal response to sheep red blood cells in zebra finches. Oecologia 168, 355–359 (2012). https://doi.org/10.1007/s00442-011-2115-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2115-9