Abstract
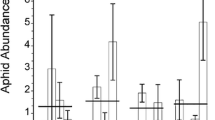
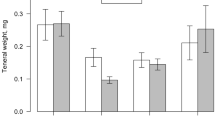
We examined how the galling aphid Pemphigus batae manipulates resource translocation patterns of resistant and susceptible narrowleaf cottonwood Populus angustifolia. Using carbon-14 (14C)-labeling experiments in common garden trials, five patterns emerged. First, although aphid galls on resistant and susceptible genotypes did not differ in their capacity to intercept assimilates exported from the leaf they occupied, aphids sequestered 5.8-fold more assimilates from surrounding leaves on susceptible tree genotypes compared to resistant genotypes. Second, gall sinks on the same side of a shoot as a labeled leaf were 3.4-fold stronger than gall sinks on the opposite side of a shoot, which agrees with patterns of vascular connections among leaves of the same shoot (orthostichy). Third, plant genetic-based traits accounted for 26% of the variation in sink strength of gall sinks and 41% of the variation in sink strength of a plant’s own bud sinks. Fourth, tree susceptibility to aphid gall formation accounted for 63% of the variation in 14C import, suggesting strong genetic control of sink–source relationships. Fifth, competition between two galls was observed on a susceptible but not a resistant tree. On the susceptible tree distal aphids intercepted 1.5-fold more 14C from the occupied leaf than did basal aphids, but basal aphids compensated for the presence of a distal competitor by almost doubling import to the gall from surrounding leaves. These findings and others, aimed at identifying candidate genes for resistance, argue the importance of including plant genetics in future studies of the manipulation of translocation patterns by phytophageous insects.






Similar content being viewed by others
References
Abrahamson WG, Weis AE (1987) Nutritional ecology of arthropod gall makers. In: Slansky F, Rodriguez JG (eds) Nutritional ecology of insects, Mites spiders, and related invertebrates. Wiley, New York, pp 235–258
Arnold TM, Schultz JC (2002) Induced sink strength as a prerequisite for induced tannin biosynthesis in developing leaves of Populus. Oecologia 130:585–593
Arnold T, Appel H, Patel V, Stocum E, Kavalier A, Schultz J (2004) Carbohydrate translocation determines the phenolic content of Populus foliage: a test of the sink-source model of plant defense. New Phytol 164:157–164
SAS Institute (2009) JMP version 8.0.1. SAS Institute, Cary
Bailey JK, Wooley SC, Lindroth RL, Whitham TG (2006) Importance of species interactions to community heritability: a genetic basis to trophic-level interactions. Ecol Lett 9:78–85
Balibrea Lara ME, Gonzalez Garcia MC, Fatima T, Ehneβ R, Lee TK, Proels R, Tanner W, Roitsch T (2004) Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 16:1276–1287
Berryman AA (1988) Toward a unified theory of plant defense. In: Mattson W, Levieux J, Bernard-Dagan C (eds) Mechanisms of woody plant defenses against insects. Springer, Berlin, pp 39–55
Billett EE, Burnett JH (1978) The host parasite physiology of the maize smut fungus Ustilago maydis. Part II: Translocation of C-14 labeled assimilates in smutted maize plants. Physiol Plant Pathol 12:103–112
Burstein M, Wool D, Eshel A (1994) Sink strength and clone size of sympatric, gall-forming aphids. Eur J Entomol 91:57–61
Cervera MT, Storme V, Ivens B, Gusmao J, Liu BH, Hostyn V, Van Slycken J, Van Montagu M, Boerjan W (2001) Dense genetic maps of three Populus species (Populus deltoides, P nigra, and P. trichocarpa) based on AFLP and microsatellite markers. Genetics 158:787–809
Cook MG, Evans LT (1983) The role of sink size and location in the partitioning of assimilates in wheat ears. Aust J Plant Physiol 10:313–327
Crainiceanu C, Ruppert D (2004) Likelihood ratio tests in linear mixed models with one variance component. J Royal Stat Soc B 66:165–185
Development Core Team R (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Dickson RE, Nelson EA (1982) Fixation and distribution of 14C in Populus deltoides during dormancy induction. Physiol Plant 54:393–401
Dickson LL, Whitham TG (1996) Genetically-based plant resistance traits affect arthropods, fungi, and birds. Oecologia 106:400–406
Dorchin N, Cramer MD, Hoffmann JH (2006) Photosynthesis and sink activity of wasp-induced galls in Acacia pycnantha. Ecology 87:1781–1791
Elzen GW (1983) Cytokinins and insect galls. Comp Biochem Physiol 76:17–19
Evans LM, Allan GJ, Shuster SM, Woolbright SA, Whitham TG (2008) Tree hybridization and genotypic variation drive cryptic speciation of a specialist mite herbivore. Evolution 62:3027–3040
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Pearson Education, Essex
Fay PA, Hartnett DC, Knapp AK (1996) Plant tolerance of gall-insect attack and gall-insect performance. Ecology 77:521–534
Fernandes GW, Júnior PDM, Schönrogge K (2008) Plant organ abscission and the green island effect caused by gallmidges (Cecidomyiidae) on tropical trees. Arthropod Plant Interact 2:93–99
Giron D, Kaiser W, Imbault N, Casas J (2007) Cytokinin-mediated leaf manipulation by a leafminer caterpillar. Biol Lett 3:340–343
Harris P (1980) Effects of Urophora affinis (Frfld.) and U. quadrifasciata (Meig.) (Diptera: Tephritidae) on Centaurea diffusa (Lam.) and C. maculosa (Lam.) (Compositae). Zool Entomol 90:190–201
Hartley SE, Lawton JH (1992) Host-plant manipulation by gall-insects: a test of the nutrition hypothesis. J Animal Ecol 61:113–119
Inbar M, Eshel A, Wool D (1995) Interspecific competition among phloem-feeding insects mediated by induced host-plant sinks. Ecology 76:1506–1515
Jones CG, Hopper RF, Coleman JS, Krischik VA (1993) Control of systemically induced herbivore resistance by plant vascular architecture. Oecologia 93:452–456
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Karban R, Myers JH (1989) Induced plant responses to herbivory. Annu Rev Ecol Syst 20:331–348
Keith AR, Bailey JK, Whitham TG (2010) A genetic basis to community repeatability and stability. Ecology 91:3398–3406
Larson PR, Isebrands JG (1971) The plastochron index as applied to developmental studies of cottonwood. Can J For Res 1:1–11
Larson KC, Whitham TG (1991) Manipulation of food resources by a gall-forming aphid: the physiology of sink-source interactions. Oecologia 88:15–21
Larson KC, Whitham TG (1997) Competition between gall aphids and natural plant sinks: plant architecture affects resistance to galling. Oecologia 109:575–582
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer, Sunderland
Mapes CC, Davies PJ (2001) Cytokinins in the ball gall of Solidego altissima and in the gall forming larvae of Eurosta solidaginis. New Phytol 151:203–212
McIntyre PJ, Whitham TG (2003) Plant genotype affects long-term herbivore population dynamics and extinction: conservation implications. Ecology 84:311–322
Moran NA, Whitham TG (1988) Predicting population fluctuations of organisms with complex life cycles: an aphid example. Ecology 69:1214–1218
Nykänen H, Koricheva J (2004) Damage-induced changes in woody plants and their effects on insect herbivore performance: a meta-analysis. Oikos 104:247–268
Orians C (2005) Herbivores, vascular pathways, and systemic induction: facts and artifacts. J Chem Ecol 31:2231–2242
Rehill BJ, Schultz JC (2003) Enhanced invertase activities in the galls of Hormaphis hamamelidis. J Chem Ecol 29:2703–2720
Rohfritsch O (1988) Food supply mechanism related to gall structure with the example of Geocryta galii LW (Cecidomyiidae, Oligotrophini) on Galium mollugo L. Phytophaga 2:1–17
Roitsch T, Gonzalez MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9:606–613
Sardesai N, Rajyashri KR, Behura SK, Nair S, Mohan M (2001) Genetic, physiological and molecular interactions of rice and its major dipteran pest, gall midge. Plant Cell Tiss Organ Cult 64:115–131
Schweitzer JA, Bailey JK, Hart SC, Wimp GM, Chapman SK, Whitham TG (2005) The interaction of plant genotype and herbivory decelerate leaf litter decomposition and alter nutrient dynamics. Oikos 110:133–145
Tooker JF, Rohr JR, Abrahamson WG, De Moraes CM (2008) Gall insects can avoid and alter indirect plant defenses. New Phytol 178:657–671
Tuskan GA, DiFazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A, Schein J, Sterck L, Aerts A, Bhalerao RR, Bhalerao RP, Blaudez D, Boerjan W, Brun A, Brunner A, Busov V, Campbell M, Carlson J, Chalot M, Chapman J, Chen GL, Cooper D, Coutinho M, Couturier J, Covert S, Cronk Q, Cunningham R, Davis J, Degroeve S, Déjardin A, dePamphilis C, Detter J, Dirks B, Dubchak I, Duplessis S, Ehlting J, Ellis B, Gendler K, Goodstein D, Gribskov M, Grimwood J, Groover A, Gunter L, Hamberger B, Heinze B, Helariutta Y, Henrissat B, Holligan D, Holt R, Huang W, Islam-Faridi N, Jones S, Jones-Rhoades M, Jorgensen R, Joshi C, Kangasjärvi J, Karlsson J, Kelleher C, Kirkpatrick R, Kirst M, Kohler A, Kalluri U, Larimer F, Leebens-Mack J, Leplé JC, Locascio P, Lou Y, Lucas S, Martin F, Montanini B, Napoli C, Nelson DR, Nelson C, Nieminen K, Nilsson O, Pereda V, Peter G, Philippe R, Pilate G, Poliakov A, Razumovskaya J, Richardson P, Rinaldi C, Ritland K, Rouzé P, Ryaboy D, Schmutz J, Schrader J, Segerman B, Shin H, Siddiqui A, Sterky F, Terry A, Tsai CJ, Uberbacher E, Unneberg P, Vahala J, Wall K, Wessler S, Yang G, Yin T, Douglas C, Marra M, Sandberg G, Van de Peer Y, Rokhsar D (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313:1596–1604
van Staden J, Davey JE (1978) Endogenous cytokinins in the laminae and galls of Erythrina latissima leaves. Bot Gaz 139:36–41
Wareing PF, Patrick J (1975) Source-sink relations and the partition of assimilates in the plant. In: Cooper J (ed) Photosynthesis and productivity in different environments. Cambridge University Press, Cambridge, pp 481–499
Watson MA, Casper B (1984) Morphogenetic constraints on patterns of carbon distribution in plants. Annu Rev Ecol Syst 15:233–258
Way MJ, Cammell M (1970) Aggregation behavior in relation of food utilization by aphids. In: Watson A (ed) Animal populations in relation to their food resources. Blackwell, Oxford, pp 229–246
Weis AE, Kapelinski A (1984) Manipulation of host plant development by the gall-midge Rhabdophaga strobiloides. Ecol Entomol 9:457–465
Whitham TG (1978) Habitat selection by Pemphigus aphids in response to resource limitation and competition. Ecology 59:1164–1176
Whitham TG (1979) The territorial behavior of Pemphigus gall aphids. Nature 279:324–325
Whitham TG (1983) Host manipulation of parasites: within-plant variation as a defense against rapidly evolving pests. In: Denno RF, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 15–41
Whitham TG (1986) Costs and benefits of territoriality: behavorial and reproductive release by competing aphids. Ecology 67:139–147
Whitham TG (1989) Plant hybrid zones as sinks for pests. Science 244:1490–1493
Williams AG, Whitham TG (1986) Premature leaf abscission: an aphid induced plant defense against gall aphids. Ecology 67:1619–1627
Wool D, Aloni R, Ben-Zvi O, Wollberg M (1999) A galling aphid furnishes its home with a built-in pipeline to the host food supply. Entomol Exp Applic 91:183–186
Woolbright SA, DiFazio SP, Yin T, Martinsen GD, Zhang X, Allan GJ, Whitham TG, Keim P (2008) A dense linkage map of hybrid cottonwood (Populus fremontii × P. angustifolia) contributes to long-term ecological research and comparison mapping in a model forest tree. Heredity 100:59–70
Wu A, Thrower LB (1981) The physiological association between Aphis craccivora (Koch) and Vigna sesquipedalis (Fruw.). New Phytol 88:89–102
Yin T, Zhang X, Huang M, Wang M, Zhuge Q, Tu S, Zhu L, Wu R (2002) Molecular linkage maps of the Populus genome. Genome 45:541–555
Zhu J, Oger PM, Schrammeijer B, Hooykaas PJJ, Farrand SK, Winans SC (2000) The bases of crown gall tumorigenesis. J Bact 182:3885–3895
Acknowledgments
We are especially grateful to R. Dickson, J. Isebrands, and P. Larson of the Forest Service Laboratory in Rhinelander, WI, for sharing with us their 14C-labeling techniques on eastern cottonwood and providing critical advice for this study. We thank D. Kimberling, L. Von der Heydt, and R. Pantera for assistance in the laboratory and T. Craig, G. Fernandez, D. Gettinger, J. Itami, M. Kearsley, N. Moran, and C. Sacchi for advice during the study. R. Foust provided laboratory space. This research was supported by the National Science Foundation, Sigma Xi, the Bilby Research Center and Organized Research of Northern Arizona University, the Merriam-Powell Center for Environmental Research, and the Utah Power and Light Co.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Colin Orians.
Rights and permissions
About this article
Cite this article
Compson, Z.G., Larson, K.C., Zinkgraf, M.S. et al. A genetic basis for the manipulation of sink–source relationships by the galling aphid Pemphigus batae . Oecologia 167, 711–721 (2011). https://doi.org/10.1007/s00442-011-2033-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2033-x