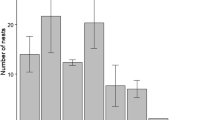
Abstract
Generally in birds, the classic sex roles of male competition and female choice result in females providing most offspring care while males face uncertain parentage. In less than 5% of species, however, reversed courtship sex roles lead to predominantly male care and low extra-pair paternity. These role-reversed species usually have reversed sexual size dimorphism and polyandry, confirming that sexual selection acts most strongly on the sex with the smaller parental investment and accordingly higher potential reproductive rate. We used parentage analyses and observations from three field seasons to establish the social and genetic mating system of pheasant coucals, Centropus phasianinus, a tropical nesting cuckoo, where males are much smaller than females and provide most parental care. Pheasant coucals are socially monogamous and in this study males produced about 80% of calls in the dawn chorus, implying greater male sexual competition. Despite the substantial male investments, extra-pair paternity was unusually high for a socially monogamous, duetting species. Using two or more mismatches to determine extra-pair parentage, we found that 11 of 59 young (18.6%) in 10 of 21 broods (47.6%) were not sired by their putative father. Male incubation, starting early in the laying sequence, may give the female opportunity and reason to seek these extra-pair copulations. Monogamy, rather than the polyandry and sex-role reversal typical of its congener, C. grillii, may be the result of the large territory size, which could prevent females from monopolising multiple males. The pheasant coucal’s exceptional combination of classic sex-roles and male-biased care for extra-pair young is hard to reconcile with current sexual selection theory, but may represent an intermediate stage in the evolution of polyandry or an evolutionary remnant of polyandry.




Similar content being viewed by others
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Andersson M (1995) Evolution of reversed sex roles, sexual size dimorphism, and mating system in coucals (Centropodidae, Aves). Biol J Linn Soc 54:173–181
Andersson M (2005) Evolution of classical polyandry: three steps to female emancipation. Ethology 111:1–23
Andersson M, Norberg RA (1981) Evolution of reversed sexual size dimorphism and role partitioning among predatory birds, with a size scaling of flight performance. Biol J Linn Soc 15:105–130
Birkhead TR, Møller AP (1992) Sperm competition in birds: evolutionary causes and consequences. Academic, London
Blanckenhorn WU (2005) Behavioral causes and consequences of sexual size dimorphism. Ethology 111:977–1016
Brohede J, Primmer CR, Møller A, Ellegren H (2002) Heterogeneity in the rate and pattern of germline mutation at individual microsatellite loci. Nucleic Acids Res 30:1997–2003
Butchart SHM, Seddon N, Ekstrom JMM (1999) Polyandry and competition for territories in bronze-winged jacanas. J Anim Ecol 68:928–939
Calder WAI (1967) Breeding behavior of the roadrunner, Geococcyx californianus. Auk 84:597–598
Carlson A (1989) Courtship feeding and clutch size in red-backed shrikes (Lanius collurio). Am Nat 133:454–457
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Clutton-Brock TH, Parker GA (1992) Potential reproductive rates and the operation of sexual selection. Q Rev Biol 67:437–456
Clutton-Brock TH, Vincent ACJ (1991) Sexual selection and the potential reproductive rates of males and females. Nature 351:58–60
Cockburn A (2006) Prevalence of different modes of parental care in birds. Proc Biol Sci 273:1375–1383
Colwell MA, Oring LW (1989) Extra-pair mating in the spotted sandpiper: a female mate acquisition tactic. Anim Behav 38:675–684
Darwin C (1871) The descent of man and selection in relation to sex. John Murray, London
Dawkins R, Carlisle TR (1976) Parental investment, mate desertion and a fallacy. Nature 262:131–133
Delehanty DJ, Fleischer RC, Colwell MA, Oring LW (1998) Sex-role reversal and the absence of extra-pair fertilization in Wilson’s phalaropes. Anim Behav 55:995–1002
Emlen ST, Wrege PH (2004) Size dimorphism, intrasexual competition, and sexual selection in wattled jacana (Jacana jacana), a sex-role-reversed shorebird in Panama. Auk 121:391–403
Geberzahn N, Goymann W, Muck C, ten Cate C (2009) Females alter their song when challenged in a sex-role reversed bird species. Behav Ecol Sociobiol 64:193–204
Gill SA, Vonhof MJ, Stutchbury BJ, Morton ES, Quinn JS (2005) No evidence for acoustic mate-guarding in duetting buff-breasted wrens (Thryothorus leucotis). Behav Ecol Sociobiol 57:557–565
Goymann W, Wingfield JC (2004) Competing females and caring males. Sex steroids in African black coucals, Centropus grillii. Anim Behav 68:733–740
Goymann W, Wittenzellner A, Wingfield J (2004) Competing females and caring males. Polyandry and sex-role reversal in African black coucals, Centropus grillii. Ethology 110:807–823
Goymann W, Kempenaers B, Wingfield J (2005) Breeding biology, sexually dimorphic development and nestling testosterone concentrations of the classically polyandrous African black coucal, Centropus grillii. J Ornithol 146:314–324
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Hall ML (2004) A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 55:415–430
Hall ML (2009) A review of vocal duetting in birds. Adv Stud Behav 40:67–121
Hall ML, Magrath RD (2000) Duetting and mate-guarding in Australian magpie-larks (Grallina cyanoleuca). Behav Ecol Sociobiol 47:180–187
Heinsohn R, Legge S, Endler JA (2005) Extreme reversed sexual dichromatism in a bird without sex role reversal. Science 309:617–619
Hicks RK, Restall R (1992) Pheasant coucal Centropus phasianinus attacking birds caught in a mist net. Muruk 5:143
Higgins PJ (1999) Handbook of Australian, New Zealand and Antarctic birds. Volume 4: parrots to Dollarbirds. Oxford University Press, Melbourne
Jenni DA (1974) Evolution of polyandry in birds. Am Zool 14:129–144
Jones RC, Lin M (1993) Spermatogenesis in birds. Oxf Rev Reprod Biol 15:234–264
Klump GM (1996) Bird communication in a noisy world. In: Kroodsma DE, Miller EH (eds) Ecology and evolution of acoustic communication in birds. Cornell University press, Ithaca, pp 321–338
Kokko H, Jennions M (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Langmore NE (1998) Functions of duet and solo songs of female birds. Trends Ecol Evol 13:136–140
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Maurer G (2007a) Ecology and evolution of sex-roles in the pheasant coucal Centropus phasianinus. Australian National University, Canberra
Maurer G (2007b) Love is in the air: arboreal copulations in the pheasant coucal Centropus phasianinus. North Territory Nat 19:48–50
Maurer G (2008) Who cares? Males provide most parental care in a monogamous nesting cuckoo. Ethology 114:540–547
Maurer G, Hale ML, Verduijn MH, Wolff K (2005) Polymorphic microsatellite loci in pheasant coucal (Centropus phasianinus). Mol Ecol Notes 5:337–339
Maurer G, Smith C, Süsser M, Magrath RD (2008) Solo and duet calling in the pheasant coucal: sex and individual call differences in a nesting cuckoo with reversed sexual size dimorphism. Aust J Zool 56:143–149
Maurer G, Beck N, Double MC (2009) A ‘feather-trap’ for collecting DNA samples from birds. Mol Ecol Resour 10:129–134
Maynard Smith J (1977) Parental investment: a prospective analysis. Anim Behav 25:1–9
Maynard Smith J (1978) The evolution of sex. Cambridge University Press, Cambridge
Michalek KG, Winkler H (2001) Parental care and parentage in monogamous great spotted woodpeckers (Picoides major) and middle spotted woodpeckers (Picoides medius). Behaviour 138:1259–1285
Muck C, Kempenaers B, Kuhn S, Valcu M, Goymann W (2009) Paternity in the classical polyandrous black coucal (Centropus grillii)—a cuckoo accepting cuckoldry? Behav Ecol 20:1185–1193
Nicholls JA, Double MC, Rowell DM, Magrath RD (2000) The evolution of cooperative and pair breeding in thornbills Acanthiza (Pardalotidae). J Avian Biol 31:165–176
Nisbet ICT (1973) Courtship-feeding, egg-size and breeding success in common terns. Nature 241:141
Oring LW, Lank DB (1986) Polyandry in spotted sandpipers: the impact of environment and experience. In: Rubenstein DR, Wrangham RW (eds) Ecological aspects of social evolution. Princeton University Press, Princeton, pp 21–42
Oring LW, Fleischer RC, Reed JM, Marsden KE (1992) Cuckoldry through stored sperm in the sequentially polyandrous spotted sandpiper. Nature 359:631–633
Owens IPF (2002) Male-only care and classical polyandry in birds: phylogeny, ecology and sex differences in remating opportunities. Philos Trans R Soc Lond B 357:283–293
Payne RB (2005) Bird families of the world: the Cuckoos. Oxford University Press, Oxford
Petrie M, Kempenaers B (1998) Extra-pair paternity in birds: explaining variation between species and populations. Trends Ecol Evol 13:52–58
Pierce EP, Lifjeld JT (1998) High paternity without paternity assurance behaviour in the purple sandpiper, a species with high paternal investment. Auk 115:602–612
Rahn H, Paganelli CV, Ar A (1975) Relation of avian egg weight to body weight. Auk 92:750–765
Ralph CP (1975) Life style of Coccyzus pumilus, a tropical cuckoo. Condor 77:60–72
Sheldon B (2002) Relating paternity to paternal care. Philos Trans R Soc Lond 357:341–350
Slotow R (1996) Black coucal, Centropus grillii, egg volume predicts their polyandrous mating system. J Avian Biol 27:171–173
Staicer CA, Spector DA, Horn AG (1996) The dawn chorus and other diel patterns in acoustic signalling. In: Kroodsma DE, Miller EH (eds) Ecology and evolution of acoustic comminucation in birds. Cornell University Press, Ithaca
Szekely T, Reynolds JD, Figuerola J (2000) Sexual size dimorphism in shorebirds, gulls, and alcids: the influence of sexual and natural selection. Evolution 54:1404–1413
Taplin A, Beurteaux Y (1992) Aspects of the breeding biology of the pheasant coucal, Centropus phasianinus. Emu 92:141–146
Trivers RL (1972) Parental investment and sexual selection. In: Cambell B (ed) Sexual selection and the descent of man. Aldine, Chicago, pp 136–179
Vernon CJ (1971) Notes on the biology of the black coucal. Ostrich 42:242–258
Vincent A, Ahnesjo I, Berglund A, Rosenqvist G (1992) Pipefishes and seahorses: are they all sex role reversed? Trends Ecol Evol 7:237–241
Voigt C, Goymann W (2007) Sex-role reversal is reflected in the brain of African black coucals (Centropus grillii). Dev Neurobiol 67:1560–1573
Weising K, Wolff K, Nybom H, Meyer W (1995) DNA fingerprinting in plants and fungi. CRC, Boca Raton
Westneat DF, Sherman PW (1993) Parentage and the evolution of parental behavior. Behav Ecol 4:66–77
Whitfield DP (1995) Behaviour and ecology of a polyandrous population of grey phalaropes Phalaropus fulicarius in Iceland. J Avian Biol 26:349–352
Wright J (1998) Paternity and paternal care. In: Møller AP, Birkhead TR (eds) Sperm competition and sexual selection. Academic, San Diego, pp 117–145
Acknowledgments
We thank S. Musgrave, A. Quellmalz, S. Quinlan, C. Smith, M. Starling, R. Noske and W. Goymann with planning and conducting the fieldwork, and N. Beck, S. Cooney, N. Langmore and two anonymous reviewers for comments on earlier drafts. The study received support from the Stuart Leslie Bird Research Award, the Cayley Memorial Scholarship, the Ingram trust, and The North Australia Research Unit. The work was conducted under permits from Parks Northern Territory (16973) and the Ethics Committee of the Australian National University (F.BTZ.56.03) and complies with the laws and regulations of the Commonwealth of Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Douglas Robinson.
Rights and permissions
About this article
Cite this article
Maurer, G., Double, M.C., Milenkaya, O. et al. Breaking the rules: sex roles and genetic mating system of the pheasant coucal. Oecologia 167, 413–425 (2011). https://doi.org/10.1007/s00442-011-2002-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2002-4