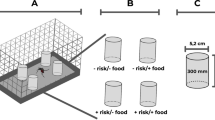
Abstract
A host’s first line of defense in response to the threat of parasitic infection is behavior, yet the efficacy of anti-parasite behaviors in reducing infection are rarely quantified relative to immunological defense mechanisms. Larval amphibians developing in aquatic habitats are at risk of infection from a diverse assemblage of pathogens, some of which cause substantial morbidity and mortality, suggesting that behavioral avoidance and resistance could be significant defensive strategies. To quantify the importance of anti-parasite behaviors in reducing infection, we exposed larval Pacific chorus frogs (Pseudacris regilla) to pathogenic trematodes (Ribeiroia and Echinostoma) in one of two experimental conditions: behaviorally active (unmanipulated) or behaviorally impaired (anesthetized). By quantifying both the number of successful and unsuccessful parasites, we show that host behavior reduces infection prevalence and intensity for both parasites. Anesthetized hosts were 20–39% more likely to become infected and, when infected, supported 2.8-fold more parasitic cysts. Echinostoma had a 60% lower infection success relative to the more deadly Ribeiroia and was also more vulnerable to behaviorally mediated reductions in transmission. For Ribeiroia, increases in host mass enhanced infection success, consistent with epidemiological theory, but this relationship was eroded among active hosts. Our results underscore the importance of host behavior in mitigating disease risk and suggest that, in some systems, anti-parasite behaviors can be as or more effective than immune-mediated defenses in reducing infection. Considering the severe pathologies induced by these and other pathogens of amphibians, we emphasize the value of a broader understanding of anti-parasite behaviors and how co-occurring stressors affect them.




Similar content being viewed by others
References
Anderson RM, May RM (1982) The population biology of infectious disease. Springer, Berlin
Baker RL, Smith BP (1997) Conflict between antipredator and anti-parasite behaviour in larval damselflies. Oecologia 109:622–628
Barber I, Hoare D, Krause J (2000) Effects of parasites on fish behaviour: a review and evolutionary perspective. Rev Fish Biol Fish 10:131–165
Bridges CM (1999) Effects of a pesticide on tadpole activity and predator avoidance behavior. J Herpetol 33:303–306
Bush AK, Lafferty D, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83: 575–583
Collins JP, Crump ML (2009) Extinction in our times: global amphibian decline. Oxford University Press, Oxford
Decaestecker E, De Meester L, Ebert D (2002) In deep trouble: habitat selection constrained by multiple enemies in zooplankton. Proc Natl Acad Sci USA 99:5481–5485
Detwiler JT, Minchella DJ (2009) Intermediate host availability masks the strength of experimentally-derived colonization patterns in echinostome trematodes. Int J Parasitol 39:585–590
Ezenwa VO (2004) Selective defecation and selective foraging: antiparasite behavior in wild ungulates? Ethology 110:851–862
Fried B, Pane PL, Reddy A (1997) Experimental infection of Rana pipiens tadpoles with Echinostoma trivolvis cercariae. Parasitol Res 83:666–669
Gosner N (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hall SR, Lafferty KD, Brown JM, Caceres CE, Chase JM, Dobson AP, Holt RD, Jones CG, Randolph SE, Rohani P (2008) Is infectious disease just another type of predator-prey interaction? In: Ostfeld RS, Keesing F, Eviner VT (eds) Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems. Princeton University Press, New Jersey
Hart BL (1990) Behavioral adaptations to pathogens and parasites: 5 strategies. Neurosci Biobehav Rev 14:273–294
Hart BL (1992) Behavioral adaptations to parasitism: an ethological approach. J Parasitol 78:256–265
Hart BL (1994) Behavioral defense against parasites: interaction with parasite invasiveness. Parasitology 109:S139–S151
Holland MP, Skelly DK, Kashgarin M, Bolden SR, Harrison LM, Cappello M (2007) Echinostome infection in green frogs (Rana clamitans) is stage and age dependent. J Zool 271:455–462
Holt RD, Dobson AP (2006) Extending the principles of community ecology to address the epidemiology of host-pathogen systems. In: Collinge SK, Ray C (eds) Disease ecology: community structure and pathogen dynamics. Oxford University Press, Oxford
Johnson PTJ, Hartson RB (2009) All hosts are not equal: explaining differential patterns of malformations in an amphibian community. J Anim Ecol 78:191–201
Johnson PTJ, McKenzie MJ (2008) Effects of environmental change on helminth infections in amphibians: exploring the emergence of Ribeiroia and Echinostoma infections in North America. In: Fried B, Toledo R (eds) The biology of echinostomes. Springer, New York, pp 249–280
Johnson PTJ, Lunde KB, Ritchie EG, Launer AE (1999) The effect of trematode infection on amphibian limb development and survivorship. Science 284:802–804
Johnson PTJ, Lunde KB, Haight RW, Bowerman J, Blaustein AR (2001) Ribeiroia ondatrae (Trematoda: digenea) infection induces severe limb malformations in western toads (Bufo boreas). Can J Zool 79:370–379
Johnson PTJ, Preu ER, Sutherland DR, Romansic J, Han B, Blaustein AR (2006) Adding infection to injury: synergistic effects of predation and parasitism on salamander limb malformations. Ecology 87:2227–2235
Johnson PTJ, Chase JM, Dosch KL, Gross J, Hartson RB, Larson D, Sutherland DR, Carpenter SR (2007) Aquatic eutrophication promotes pathogenic infection in amphibians. Proc Natl Acad Sci USA 104:15781–15786
Karvonen A, Seppala O, Valtonen ET (2004) Parasite resistance and avoidance behaviour in preventing eye fluke infections in fish. Parasitology 129:159–164
Kiesecker JM (2002) Synergism between trematode infection and pesticide exposure: a link to amphibian deformities in nature? Proc Natl Acad Sci USA 99:9900–9904
Kiesecker JM, Skelly DK (2000) The choice of oviposition site by gray treefrogs: the role of potential parasitic infection. Ecology 81:2939–2943
Kiesecker JM, Skelly DK, Beard KH, Preisser E (1999) Behavioral reduction of infection risk. Proc Natl Acad Sci USA 96:9165–9168
Koprivnikar J, Forbes MR, Baker RL (2006) On the efficacy of anti-parasite behaviour: a case study of tadpole susceptibility to cercariae of Echinostoma trivolvis. Can J Zool 84:1623–1629
Koprivnikar J, Forbes MR, Baker RL (2007) Contaminant effects on host parasite interactions: atrazine, frogs, and trematodes. Environ Toxicol Chem 26:2166–2170
Kuris AM, Hechinger RF, Shaw JC, Whitney KL, Aguirre-Macedo L, Boch CA, Dobson AP, Dunham EJ, Fredensborg BL, Huspeni TC, Lorda J, Mababa L, Mancini FT, Mora AB, Pickering M, Talhouk NL, Torchin ME, Lafferty KD (2008) Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454:515–518
Lafferty KD, Holt RD (2003) How should environmental stress affect the population dynamics of disease? Ecol Lett 6:654–664
Lafferty KD, Kuris AM (2002) Trophic strategies, animal diversity and body size. Trends Ecol Evol 17:507–513
Lafferty KD, Allesina S, Arim M, Cherie J, et al (2008) Parasites in food webs: the ultimate missing links. Ecol Lett 11:533–546
Lass S, Spaak P (2003) Chemically induced anti-predator defences in plankton: a review. Hydrobiologia 491:221–239
Marco A, Quilchano C, Blaustein AR (1999) Sensitivity to nitrate and nitrite in pond breeding amphibians from the Pacific Northwest, USA. Environ Toxicol Chem 18:2836–2839
Moore J (2002) Parasites and the behavior of animals. Oxford University Press, New York
Mooring MS, Blumstein DT, Stoner CJ (2004) The evolution of parasite-defence grooming in ungulates. Biol J Linn Soc 81:17–37
Preisser EL, Bolnick DI, Benard MF (2005) Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86:501–509
Prentice MA (1984) A field-evolved differential filtration method for recovery of schistosome cercariae. Ann Trop Med Parasitol 78:117–127
Råberg L, Graham AL, Read AF (2009) Decomposing health: tolerance and resistance to parasites in animals. Phil Trans Roy Soc B 364:37–49
Raffel TR, Martin LB, Rohr JR (2008) Parasites as predators: unifying natural enemy ecology. Trends Ecol Evol 23:610–618
Relyea RA (2001a) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Relyea RA (2001b) The relationship between predation risk and antipredator responses in larval anurans. Ecology 82:541–554
Relyea RA, Mills N (2001) Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor). Proc Natl Acad Sci USA 98:2491–2496
Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman JT, Johnson CM, Johnson LB, Lieske C, Piwoni MD, Schoff PK, Beasley VR (2008a) Agrochemicals increase trematode infections in a declining amphibian species. Nature 455:1235–1239
Rohr JR, Raffel TR, Sessions SK, Hudson PJ (2008b) Understanding the net effects of pesticides on amphibian trematode infections. Ecol Appl 18:1743–1753
Rohr JR, Swan A, Raffel TR, Hudson PJ (2009) Parasites, info-disruption, and the ecology of fear. Oecologia 159:447–454
Rohr JR, Raffel TR, Sessions SK (2010) Digenetic trematodes and their relationship to amphibian declines and deformities. In: H Heatwole (ed) Amphibian biology, vol 8: amphibian decline: diseases, parasites, maladies, and pollution. J.W. Surrey Beatty & Sons, Chipping Norton
Schotthoefer AM, Cole RA, Beasley VR (2003) Relationship of tadpole stage to location of echinostome cercariae encystment and the consequences for tadpole survival. J Parasitol 89:475–482
Sessions SK, Ruth SB (1990) Explanation for naturally occurring supernumerary limbs in amphibians. J Exp Zool 254:38–47
Skelly DK (1994) Activity level and the susceptibility of anuran larvae to predation. Anim Behav 47:465–468
Skerrat LF, Berger L, Speare R (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134
Stopper GF, Hecker L, Franssen RA, Sessions SK (2002) How trematodes cause limb deformities in amphibians. J Exp Zool 294:252–263
Sutherland DR (2005) Parasites of North American Frogs. In: Lannoo MJ (ed) Amphibian declines: the conservation status of United States species. University of California Press, Berkeley, pp 109–123
Szuroczki D, Richardson JML (2009) The role of trematode parasites in larval anuran communities: an aquatic ecologist’s guide. Oecologia 161:371–385
Taylor SK, Williams ES, Mills KW (1999) Effects of malathion on disease susceptibility in Woodhouse’s toads. J Wildlife Dis 35:536–541
Taylor CN, Oseen KL, Wassersug RJ (2004) On the behavioural response of Rana and Bufo tadpoles to Echinostomatoid cercariae: implications to synergistic factors influencing trematode infections in anurans. Can J Zool 82:701–706
Thiemann GW, Wassersug RJ (2000) Patterns and consequences of behavioural responses to predators and parasites in Rana tadpoles. Biol J Linn Soc 71:513–528
Wake DB, Vredenburg VT (2008) Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA105:11466–11473
Werner EE, Peacor SC (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84:1083–1100
Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, Blakeslee AMH (2007) Parasites alter community structure. Proc Natl Acad Sci USA 104:9335–9339
Acknowledgments
We thank D. Miller, S. Collinge, S. Paull, S. Orlofske, K. Dosch, and R. Jadin for their guidance and assistance in this project. S. Kupferberg and the Angelo Reserve generously provided experimental materials. EWD gratefully acknowledges funding support from the University of Colorado Undergraduate Research Opportunities Program. This project was supported by a grant from the National Science Foundation (DEB-0553768) and a fellowship from the David and Lucile Packard Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Carla Caceres.
Rights and permissions
About this article
Cite this article
Daly, E.W., Johnson, P.T.J. Beyond immunity: quantifying the effects of host anti-parasite behavior on parasite transmission. Oecologia 165, 1043–1050 (2011). https://doi.org/10.1007/s00442-010-1778-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1778-y