Abstract
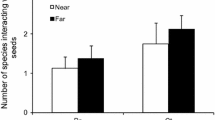
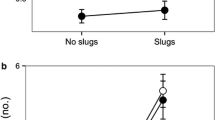
The microhabitat in which plants grow affects the outcome of their interactions with animals, particularly non-specialist consumers. Nevertheless, as most research on this topic has dealt with either mutualists or antagonists, little is known about the indirect effects of plant microhabitats on the outcome of tripartite interactions involving plants and both mutualists (e.g. seed dispersers) and antagonists (e.g. granivores). During three consecutive years, we analysed small-scale variations in the interaction of a perennial myrmecochore, Helleborus foetidus, with its seed dispersers and consumers as a function of the intensity of plant cover. Most seeds were released during the day and were rapidly removed by ants. Nevertheless, the proportion of ant-removed seeds was higher for plants located in open microhabitats than for plants surrounded by dense vegetation and rocky cover. Ant sampling revealed that seed removers were equally abundant, irrespective of the level of cover. By contrast, a few tiny ant species that feed on the reward without transporting the seeds were more abundant in highly covered microhabitats, irrespective of hellebore diaspore availability. These “cheaters” decrease the chance of removal by removers and increase the probability of seeds remaining on the ground until night, when granivore mice Apodemus sylvaticus become active. Mice also preferred foraging in covered microhabitats, where they consumed a larger proportion of seeds. Therefore, the density of cover indirectly increased seed predation risk by attracting more seed predators and cheater ants that contribute to increase seed availability for seed predators. Our results emphasize the importance of considering the indirect effects of plant microhabitat on their dispersal success. They highlight the indirect effect of cheaters that are likely to interfere in mutualisms and may lead to their collapse unless external factors such as spatio-temporal heterogeneity in seed availability constrain their effect.




Similar content being viewed by others
References
Alcántara JM, Rey PJ, Manzaneda AJ, Boulay R, Ramírez JM, Fedriani JM (2007) Geographic variation in the adaptive landscape for seed size at dispersal of the myrmecochorous Helleborus foetidus. Evol Ecol 21:411–430
Andersen AN (1988) Dispersal distance as a benefit of myrmecochory. Oecologia 75:507–511
Bas JM, Oliveras J, Goméz C (2009) Myrmecochory and short-term seed fate in Rhamnus alaternus: ant species and seed characteristics A. Oecology (in press)
Benkman CW, Holimon WC, Smith JW (2001) The influence of a competitor on the geographic mosaic of coevolution between crossbills and lodgepole pine. Evolution 55:282–294
Benkman CW, Parchman TL, Favis A, Siepielski AM (2003) Reciprocal selection causes a coevolutionary arms race between crossbills and lodgepole pine. Am Nat 162:182–194
Böhning-Gaese K, Gaese BH, Rabemanatsoa SB (1999) Importance of primary and secondary seed dispersal in the Malagasy tree Comniphora guillaumini. Ecology 80:821–832
Boulay R, Fedriani JM, Manzaneda AJ, Cerdá X (2005) Indirect effects of alternative food resources in an ant–plant interaction. Oecologia 144:72–79
Boulay R, Coll-Toledano J, Cerdá X (2006) Geographic variations in Helleborus foetidus elaiosome lipid composition: implications for dispersal by ants. Chemoecology 16:1–7
Boulay R, Carro F, Soriguer RC, Cerdá X (2007a) Synchrony between fruit maturation and effective dispersers’ foraging activity increases seed protection against seed predators. Proc R Soc Lond B 274:2515–2522
Boulay R, Cerdá X, Simon T, Roldan M, Hefetz A (2007b) Intraspecific competition in the carpenter ant Camponotus cruentatus: should we expect the Dear Enemy Effect? Anim Behav 74:985–993
Bronstein JL (1994) Conditional outcomes in mutualistic interactions. Trends Ecol Evol 9:214–217
Carro F, Pérez-Aranda D, Lamosa A, Schmalenberger HP, Pardavila X, Soriguer RC (2007) Indice de capturas y tipo de trampa: ¿qué trampa es para capturar micromamiferos? Galemys 19:73–81
Casper BB (1987) Spatial patterns of seed dispersal and postdispersal seed predation of Cryptantha flava (Boraginaceae). Am J Bot 74:1646–1655
Cerdá X, Retana J, Cros S (1997) Thermal disruption of transitive hierarchies in Mediterranean ant communities. J Anim Ecol 66:363–374
Crawley MJ (2007) The R book. Wiley, New York
Detrain C, Tasse O (2000) Seed drops and caches by the harvester ant Messor barbarus: do they contribute to seed dispersal in Mediterranean grasslands? Naturwissenschaften 87:373–376
Edut S, Eilam D (2003) Rodents in open space adjust their behavioral response to the different risk levels during barn-owl attack. BMC Ecol 3:1–16
Fedriani JM, Boulay R (2006) Foraging by fearful frugivores: combined effects of fruit ripening and predation risk. Funct Ecol 20:1070–1079
Fedriani JM, Rey PJ, Garrido JL, Guitián J, Herrera CM, Medrano M, Sánchez-Lafuente AM, Cerdá X (2004) Geographical variation in the potential of mice to constrain an ant–seed dispersal mutualism. Oikos 105:181–191
García-Castaño JL, Kollman J, Jordano P (2006) Spatial variation of post-dispersal seed removal by rodents in highland microhabitats of Spain and Switzerland. Seed Sci Res 16:213–222
Garrido JL, Rey P, Cerdá X, Herrera C (2002) Geographic variation of diaspore trati of an ant-dispersed plant (Helleborus foetidus): are ant community and diaspore trait correlated. J Ecol 90:446–455
Giladi I (2006) Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos 112:481–492
Gómez C, Espadaler X (1998) Aphaenogaster senilis Mayr (Hymenoptera, Formicidae): a possible parasite in the Myrmecochory of Euphorbia characias. Sociobiology 32:441–450
Gorb SN, Gorb E (1999) Dropping rates of elaiosome-bearing seeds during transport by ants (Formica polyctena Foerst.): implications for distance dispersal. Acta Oecol 20:509–518
Gorb E, Gorb S (2003) Seed dispersal by ants in a deciduous forest ecosystem. Kluwer, Dordrecht
Heithaus ER (1981) Seed predation by rodents on three ant-dispersed plants. Ecology 62:136–145
Herre EA, Knowlton N, Mueller U, Rehner S (1999) The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol Evol 14:49–53
Herrera CM (2002) Seed dispersal by vertebrates. In: Herrera CM, Pellmyr O (eds) Plant–animal interactions. Blackwell, Oxford, pp 185–208
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Syst 13:201–228
Hughes L, Westoby M (1992) Fate of seeds adapted for dispersal by ants in Australian sclerophyll vegetation. Ecology 73:1285–1299
Hulme PE (1994) Post-dispersal seed predation in grassland: its magnitude and sources of variation. J Ecol 82:645–652
Hulme PE (1997) Post-dispersal seed predation and the establishment of vertebrate dispersed plants in Mediterranean scrublands. Oecologia 111:91–98
Hulme PE, Borelli T (1999) Variability in post-dispersal seed predation in deciduous woodland: relative importance of location, seed species, burial and density. Plant Ecol 145:149–156
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Lortie CJ, Ganey DT, Kotler BP (2000) The effects of gerbil foraging on the natural seedbank and consequences on the annual plant community. Oikos 90:399–407
Majer JD, Lamont BB (1985) Removal of seed of Grevillea pteridifolia (Proteaceae) by ants. Austr J Bot 33:611–618
Manzaneda AJ, Fedriani JM, Rey PJ (2005) Adaptive advantages of myrmecochory: the predator-avoidance hypothesis tested over a wide geographic range. Ecography 28:583–592
Manzaneda AJ, Rey P, Boulay R (2007) Geographic and temporal variations in the ant–seed dispersal assemblage of the perennial herb Helleborus foetidus. Biol J Linn Soc 92:135–150
Mark S, Olesen JM (1996) Importance of elaiosome size to removal of ant-dispersal seeds. Oecologia 107:95–101
Ness JH (2004) Forest edges and fire ants alter the seed shadow of an ant-dispersed plant. Oecologia 138:448–454
O’Dowd DJ, Hay ME (1980) Mutualism between harvester ants and a desert ephemeral: seed escape from rodents. Ecology 61:531–540
Oberrath R, Böhning-Gaese K (2002) Phenological adaptation of ant-dispersed plants to seasonal variation in ant activity. Ecology 83:1412–1420
Ohara M, Higashi S (1987) Interference by ground beetles with the dispersal by ants of seeds of Trillium species (Liliaceae). J Ecol 75:1091–1098
Ohkawara K, Higashi S, Ohara M (1996) Effects of ants, ground beetles and the seed-fall patterns on myrmecochory of Erythronium japonicum Decne. (Liliaceae). Oecologia 106:500–506
Oliveras J, Gómez C, Bas JM, Espadaler X (2008) Mechanical defence in seeds to avoid predation by a granivorous ant. Naturwissenschaften 95:501–506
Pizo MA, Oliveira PS (1998) Interaction between ants and seeds of a nonmyrmecochorous neotropical tree, Cabralea canjerana (Meliaceae), in the Atlantic forest of southeast Brazil. Am J Bot 85:669–674
Prinzing A, Dauber J, Hammer EC, Hammouti N, Böhning-Gaese K (2007) Perturbed partners: opposite responses of plant and animal mutualist guilds to inundation disturbances. Oikos 116:1299–1310
Retana J, Picó X, Rodrigo A (2004) Dual role of harvesting ants as seed predators and dispersers of a non-myrmechorous mediterranean perennial herb. Oikos 105:377–385
Rey PJ, Manzaneda AJ (2007) Geographical variation in the determinants of seed dispersal success of a myrmecochorous herb. J Ecol 95:1381–1393
Rodgerson L (1998) Mechanical defense in seeds adapted for ant dispersal. Ecology 79:1669–1677
Segraves KA, Althoff DM, Pellmyr O (2005) Limiting cheaters in mutualism: evidence from hybridization between mutualist and cheater yucca moths. Proc R Soc Lond B 272:2195–2201
Servigne P, Detrain C (2008) Ant–seed interactions: combined effects of ant and plant species on seed removal patterns. Insect Soc 55:220–230
Smallwood J, Culver DC (1979) Colony movements of some North American ants. J Anim Ecol 48:373–382
Strauss SY (1991) Indirect effects in community ecology: their definition, study and importance. Trends Ecol Evol 6:206–210
Strauss SY, Irwin RE (2004) Ecological and evolutionary consequences of multispecies plant–animal interactions. Annu Rev Ecol Evol Syst 35:435–466
Tarborelli PA, Dacar M, Giannoni SM (2003) Effect of plant cover on seed removal by rodents in the Monte Desert (Mendoza, Argentina). Aust Ecol 28:651–657
Thompson JN (1994) The coevolutionary process. The University of Chicago Press, Chicago
Thompson JN (2005) The geographic mosaic of coevolution. The University of Chicago Press, Chicago
Turnbull CL, Culver DC (1983) The timing of seed dispersal in Viola nuttallii: attraction of dispersers and avoidance of predators. Oecologia 59:360–365
Valverde T, Silvertown J (1995) Spatial variation in the seed ecology of a woodland herb (Primula vulgaris) in relation to light environment. Funct Ecol 9:942–950
Wang BC, Smith TB (2002) Closing the seed dispersal loop. Trends Ecol Evol 17:379–385
Yu DW, Wison HB, Pierce NE (2001) An empirical model of species coexistence in a spatially structured environment. Ecology 82:1761–1771
Zelikova TJ, Dunn RR, Sanders NJ (2008) Variation in seed dispersal along an elevational gradient in Great Smoky Mountains National Park. Acta Oecol 34:155–162
Acknowledgments
This work was funded by the Spanish “Ministerio de Educación y Ciencia” (project BOS2003-01536 and CGL2006-04968/BOS to X.C. and R.B.). It was conducted with the authorization of Parque Natural de la Sierra de Grazalema and Parque Natural de las Sierras Subbéticas. Accommodation at Cabra was kindly provided by the Camacho family and at Grazalema by the authority of the park. Assistance in the field was provided by E. Angulo, A. Carvajal, I. Luque, O. González, E. García Márquez, I. Villalta and M. Vonshak. We also thank J. Minett for editing assistance and Drs J. Cronin and T. Payne for constructive comments on a previous version of the manuscript. R.B. was funded by an I3P fellowship. This work complies with current Spanish laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Florian Schiestl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boulay, R., Carro, F., Soriguer, R.C. et al. Small-scale indirect effects determine the outcome of a tripartite plant–disperser–granivore interaction. Oecologia 161, 529–537 (2009). https://doi.org/10.1007/s00442-009-1404-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1404-z