Abstract
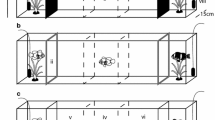
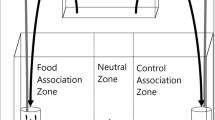
Animals use sensory stimuli to assess and select habitats, mates and food as well as to communicate with other individuals. One way they do this is to use olfaction, whereby they identify and respond to chemical cues. All organisms release odours, which mix with other chemical substances and ambient environmental conditions. The result is that animals are frequently immersed in a complex, highly dynamic sensory environment where they must identify and respond to only some of the potential stimuli they encounter in the face of significant levels of background noise. Understanding how organisms respond to different chemical cues is therefore dependent on knowing how these responses might be influenced by potential interactions with other stimuli. To test this, we examined whether the diadromous fish Galaxias maculatus was attracted to conspecific odours and whether this response differed when cues were offered in an artificial environment lacking other potential chemical stimuli (tap water) or a more natural background environment (stream water). We found that (1) fish responded to both natural stream water odours and those from conspecifics but the response to the latter was stronger; (2) the attraction to conspecific odours was stronger in tap water than in stream water, which indicates the importance of these odours may be overestimated when they are offered in artificial media. We also conducted a brief literature review, which confirmed that artificial media are commonly used in experiments and that the background environment is often not considered. Our results show that future research testing the responses of organisms to auditory, olfactory and visual cues should carefully consider the context in which cues are presented. Without doing so, such studies may inaccurately assess the importance of sensory cues in natural situations in the wild.


Similar content being viewed by others
References
Atema J (1996) Eddy chemotaxis and odour landscapes: exploration of nature with animal sensors. Biol Bulletin 191:129–138
Atema J, Kingsford MJ, Gerlach G (2002) Larval reef fish could use odour for detection, retention and orientation to reefs. Mar Ecol Prog Ser 241:151–160
Baker CF (2003) Effect of fall height and notch shape on the passage of inanga (Galaxias maculatus) and common bullies (Gobiomorphus cotidianus) over an experimental weir. N Z J Mar Freshw Res 37:283–290
Baker CF, Hicks BJ (2003) Attraction of inanga and koaro to adult odours. N Z J Mar Freshw Res 37:291–299
Barker JR, Lambert DM (1988) A genetic analysis of populations of Galaxias maculatus from the Bay of Plenty: implications for natal river return. N Z J Mar Freshw Res 22:321–326
Barnes MC, Persons MH, Rypstra AL (2002) The effect of predator chemical cue age on antipredator behavior in the Wolf spider Pardosa milvina (Araneae: Lycosidae). J Insect Behav 15:269–281
Brodin T, Johansson F, Bergsten J (2006) Predator related oviposition site selection of aquatic beetles (Hydroporus sp.) and effects on offspring life history. Freshw Biol 51:1277–1285
Brumm H, Slabbekkoorn H (2005) Acoustic communication in noise. Adv Study Behav 35:151–209
Chapman MG (2000) Poor design of behavioural experiments gets poor results: examples from intertidal habitats. J Exp Mar Bio Ecol 250:77–95
Cohen J (1988) Statistical power for the behavioral sciences, 2nd edn. Erlbaum, Hillsdale, NJ
Endler JA (1993a) Some general comments on the evolution and design of animal communication systems. Philos Trans R Soc Lond B Biol Sci 340:215–225
Endler JA (1993b) The color of light in forests and its implications. Ecol Monogr 63:1–27
Fisher HS, Wong BBM, Rosenthal GG (2006) Alteration of the chemical environment disrupts communication in a freshwater fish. Proc R Soc Lond B Biol Sci 273:526–537
Hale R (2006) An investigation of the causes of variability in settlement of diadromous galaxiids. PhD thesis. School of Anthropology, Geography and Environmental Studies/Department of Zoology, University of Melbourne, Melbourne
Hale R, Downes BJ, Swearer SE (2008) Habitat selection as a source of inter-specific differences in recruitment of two diadromous fish species. Freshw Biol 53:2145–2157
Hale R, Swearer SE (2008) Otolith microchemical and microstructural changes associated with settlement in the diadromous fish, Galaxias maculatus. Mar Ecol Prog Ser 354:229–234
Hankinson SJ, Morris MR (2003) Avoiding a compromise between sexual selection and species recognition: female swordtail fish assess multiple species-specific cues. Behav Ecol 14:282–287
Hazlett BA, Acquistapace P, Gheradi F (2006) Responses of the crayfish Orconectes virilis to chemical cues depend on flow conditions. J Crust Biol 26:94–98
Hirvonen H, Ranta E, Piironen J, Laurila A, Peuhkuri N (2000) Behavioural responses of naive Arctic charr young to chemical cues from salmonid and non-salmonid fish. Oikos 88:191–199
Johnstone RA (1996) Multiple displays in animal communication: “back-up signals” and “multiple messages”. Philos Trans R Soc Lond B Biol Sci 351:329–338
McClennan DA, Ryan MJ (1997) Responses to conspecific and heterospecific olfactory cues in the swordtail Xiphophorus cortezi. Anim Behav 54:1077–1088
McDowall RM, Charteris SC (2006) The possible advantages of terrestrial egg deposition in some fluvial diadromous galaxiid fishes (Teleosti: Galaxiidae). Fish Fish (Oxf) 7:153–164
McDowall RM, Eldon GA (1980) The ecology of whitebait migrations (Galaxiidae: Galaxias sp.). Fish Res Bull 20:1–110
McDowall RM, Mitchell CP, Brothers EB (1994) Age at migration from the sea of juvenile Galaxias in New Zealand (Pisces: Galaxiidae). Bull Mar Sci 54:385–402
Merkens M, Harestad AS, Sullivan TP (1991) Cover and efficacy of predator-based repellents for Townsend’s vole, Microtus townsendii. J Chem Ecol 17:401–412
Moller AP, Pomiankowski A (1993) Why have birds got multiple sexual ornaments? Behav Ecol Sociobiol 32:167–176
Montgomery JC, Tolimieri N, Haine OS (2001) Active habitat selection by pre-settlement reef fishes. Fish Fish (Oxf) 2:261–277
Mumm R, Hilker M (2005) The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem Senses 30:337–343
Olabarria C, Underwood AJ, Chapman MG (2002) Appropriate experimental design to evaluate preferences for microhabitat: an example of preferences by species of microgastropods. Oecologia 132:159–166
Olsen KH, Winberg S (1996) Learning and sibling odour preference in juvenile Arctic char, Salvelinus alpinus (L.). J Chem Ecol 22:773–786
Ord TJ, Peters RA, Clucas B, Stamps JA (2007) Lizards speed up visual displays in noisy motion habitats. Proc R Soc Lond B Biol Sci 274:1057–1062
Peacor SD (2006) Behavioral response of bullfrog tadpoles to chemical cues of predation risk are affected by cue age and water source. Hydrobiologia 573:39–44
Polkinghorne CN, Olson JM, Gallaher DD, Sorensen PW (2001) Larval sea lamprey release two unique bile acids to the water at sufficient rate to produce detectable riverine pheromonal plumes. Fish Physiol Biochem 24:15–30
Scholz AT, Horrall RM, Cooper JC, Hasler AD (1975) Imprinting to chemical cues: the basis for home stream selection in salmon. Science 192:1247–1249
Skelly DK (2002) Experimental venue and estimation of interaction strength. Ecology 83:2097–2101
Skelly DK (2005) Experimental venue and estimation of interaction strength: reply. Ecology 86:1068–1071
Underwood AJ, Chapman MG, Crowe TP (2004) Identifying and understanding ecological preferences for habitat or prey. J Exp Mar Biol Ecol 300:161–187
Vrieze LA, Sorensen PW (2001) Laboratory assessment of the role of a larval pheromone and natural stream odour in spawning stream localization by migratory sea lamprey (Petromyzon marinus). Can J Fish Aquat Sci 58:2374–2385
Waters JM, Dijkstra LH, Wallis GP (2000) Biogeography of a southern hemisphere freshwater fish: how important is marine dispersal? Mol Ecol 9:1815–1821
Werner EE (1998) Ecological experiments and a research program in community ecology. In: Resetarits WJ, Bernardo J (eds) Ecological experiments: issues and perspectives. Oxford University Press, Oxford
Wilder SM, DeVito J, Persons MH, Rypstra AL (2005) The effects of moisture and heat on the efficacy of chemical cues used in predator detection by the Wolf spider Pardosa milvina (Araneae: Lycosidae). J Arachnol 33:857–861
Wiley RH (2006) Signal detection and animal communication. Adv Study Behav 36:217–247
Acknowledgments
Assistance with fieldwork was provided by N. Barbee, L. Rumpff and F. McClean. Funding support from the following sources is acknowledged: the Australian Research Council, the Holsworth Wildlife Research Fund and both the Department of Resource Management and Geography and the Faculty of Science at the University of Melbourne. We thank J. Trexler and two anonymous reviewers for comments on the manuscript. These experiments complied with current Australian law and were conducted with approval from the Animal Ethics Committee at the University of Melbourne.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Joel Trexler.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hale, R., Swearer, S.E. & Downes, B.J. Separating natural responses from experimental artefacts: habitat selection by a diadromous fish species using odours from conspecifics and natural stream water. Oecologia 159, 679–687 (2009). https://doi.org/10.1007/s00442-008-1248-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1248-y