Abstract
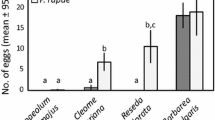
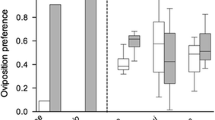
A large proportion of phytophagous insect species are specialised on one or a few host plants, and female host plant preference is predicted to be tightly linked to high larval survival and performance on the preferred plant(s). Specialisation is likely favoured by selection under stable circumstances, since different host plant species are likely to differ in suitability—a pattern usually explained by the “trade-off hypothesis”, which posits that increased performance on a given plant comes at a cost of decreased performance on other plants. Host plant specialisation is also ascribed an important role in host shift speciation, where different incipient species specialise on different host plants. Hence, it is important to determine the role of host plants when studying species divergence and niche partitioning between closely related species, such as the butterfly species pair Leptidea sinapis and Leptidea reali. In Sweden, Leptidea sinapis is a habitat generalist, appearing in both forests and meadows, whereas Leptidea reali is specialised on meadows. Here, we study the female preference and larval survival and performance in terms of growth rate, pupal weight and development time on the seven most-utilised host plants. Both species showed similar host plant rank orders, and larvae survived and performed equally well on most plants with the exceptions of two rarely utilised forest plants. We therefore conclude that differences in preference or performance on plants from the two habitats do not drive, or maintain, niche separation, and we argue that the results of this study do not support the trade-off hypothesis for host plant specialisation, since the host plant generalist Leptidea sinapis survived and performed as well on the most preferred meadow host plant Lathyrus pratensis as did Leptidea reali although the generalist species also includes other plants in its host range.




Similar content being viewed by others
References
Agrawal AA (2000) Host-range evolution: adaptation and trade-offs in fitness of mites on alternative hosts. Ecology 81:500–508
Amiet JL (2004) Ecological niche partitioning between two sympatric sibling Leptidea species (Lepidoptera, Pieridae). Rev Ecol (Terre Vie) 59:433–452
Beneš J, Konvika M, Vrabec V, Zámečník J (2003) Do the sibling species of small whites, Leptidea sinapis and L. reali (Lepidoptera, Pieridae) differ in habitat preferences? Biol Brat 58:943–951
Bernays E, Graham M (1988) On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–892
Braby MF, Truman JWH (2006) Evolution of larval host plant associations and adaptive radiation in pierid butterflies. J Evol Biol 19:1677–1690
Brooks DR, McLennan DA (2002) The nature of diversity—an evolutionary voyage of discovery. The University of Chicago Press, Chicago
Bush GL, Butlin RK (2004) Sympatric speciation in insects. In: Dieckmann U, Doebeli M, Metz JAJ, Tautz D (eds) Adaptive speciation. Cambridge University Press, Cambridge, pp 229–248
Damman H, Feeny P (1988) Mechanisms and consequences of selective oviposition by the zebra swallowtail butterfly. Anim Behav 36:563–573
Doak P, Kareiva P, Kingsolver J (2006) Fitness consequences of choosy oviposition for a time-limited butterfly. Ecology 87:395–408
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Eliasson CU, Ryrholm N, Holmer M, Jilg K, Gärdenfors U (2005) Nationalnyckeln till Sveriges flora och fauna. Fjärilar: Dagfjärilar. Hesperiidae–Nymphalidae. Artdatabanken, SLU, Uppsala
Feder JL, Forbes AA (2007) Habitat avoidance and speciation for phytophagous insect specialists. Funct Ecol 21:585–597
Feder JL, Chilote CA, Bush GL (1988) Genetic differentiation between sympatric host races of the apple maggot fly Rhagoletis pomonella. Nature 336:61–64
Feder JL, Opp SB, Wlazlo B, Reynolds K, Go W, Spisak S (1994) Host fidelity is an effective premating barrier between sympatric races of the apple maggot fly. Proc Natl Acad Sci USA 91:7990–7994
Feder JL, Reynolds K, Go W, Wang EW (1995) Intra- and interspecific competition and host race formation in the apple maggot fly, Rhagoletis pomonella (Diptera: Tephritidae). Oecologia 101:416–425
Forister ML (2004) Oviposition preference and larval performance within a diverging lineage of lycaenid butterflies. Ecol Entomol 29:264–272
Freese A, Fiedler K (2002) Experimental evidence for species distinctness of the two wood white butterfly taxa, Leptidea sinapis and L. reali (Pieridae). Nota lepid 25:39–59
Friberg M (2007) A difference in pupal morphology between the sibling species Leptidea sinapis and L. reali (Pieridae). Nota Lepid 30:61–64
Friberg M, Wiklund C (2007) Generation-dependent female choice: behavioral polyphenism in a bivoltine butterfly. Behav Ecol 18:758–763
Friberg M, Vongvanich N, Borg-Karlsson A-K, Kemp DJ, Merilaita S, Wiklund C (2008a) Female mate choice determines reproductive isolation between sympatric butterflies. Behav Ecol Sociobiol 62:873–886
Friberg M, Bergman M, Kullberg J, Wahlberg N, Wiklund C (2008b) Niche separation in space and time between two sympatric sister species—a case of ecological pleiotropy. Evol Ecol 22:1–18
Friberg M, Olofsson M, Berger D, Karlsson B, Wiklund C (2008c) Habitat choice precedes host plant choice—niche separation in a species pair of a generalist and a specialist butterfly. Oikos 117:1337–1344
Fry JD (1996) The evolution of host specialisation: are trade-offs overrated? Am Nat 148:S84–S107
Futuyma DJ (1979) Evolutionary biology, 1st edn. Sinauer, Sunderland
Futuyma DJ (2008) Sympatric speciation: norm or exception? In: Tilmon KJ (ed) Specialisation, speciation, and radiation: the evolutionary biology of herbivorous insects. University of California Press, Berkeley, pp 136–202
Futuyma DJ, Moreno G (1988) The evolution of ecological specialisation. Annu Rev Ecol Syst 19:207–234
Hunter MD, McNeil JN (1997) Host-plant quality influences diapause and voltinism in a polyphagous insect herbivore. Ecology 78:977–986
Janz N, Nyblom K, Nylin S (2001) Evolutionary dynamics of host plant specialisation: a case study of the tribe Nymphalini. Evolution 55:783–796
Janz N, Nylin S, Wahlberg N (2006) Diversity begets diversity: host expansions and the diversification of plant-feeding insects. BMC Evol Biol 6:4
Lawton JH, Strong DR Jr (1981) Community patterns and competition in folivorous insects. Am Nat 118:317–338
Lorkovic′ Z (1993) Leptidea reali REISSINGER 1989 (= lorkovicii REAL 1988), a new European species (Lepid., Pieridae). Nat Croatia 2:1–26
Martin J-F, Gilles A, Descimon H (2003) Species concepts and sibling species: the case of Leptidea sinapis and Leptidea reali. In: Boggs CL, Watt WB, Ehrlich PR (eds) Butterflies—ecology and evolution—taking flight. University of Chicago Press, Chicago, pp 459–476
Mazel R (2005) Éleménts de phylogénie dans le genre Leptidea Billberg 1820 (Lepidoptera, Pieridae, Dismorphiinae). Rev Assoc Roussill Entomol 14:98–111
Murphy SM (2004) Enemy-free space maintains swallowtail butterfly host shift. Proc Natl Acad Sci USA 101:18048–18052
Réal P (1988) Lepidoptères noveaux principalement Jurassiens. Mémoires de Comité de Liaison pour les Recherches Ecofaunistiques dans le Jura. Publication apériodique, Besançon, pp 17–24
Reissinger E (1989) Checkliste Pieride Duponchel, 1835 der Westpalaearctis (Europa, Nordwestafrika, Kaukasus, Kleinasien). Atalanta 20:149–185
Schmitz O (2007) Recent findings about the historic and current distribution of Leptidea sinapis (Linnaeus, 1758) and Leptidea reali Reissinger, 1989 (Lepidoptera, Pieridae) in the working area of the Rhenish-Westphalian Lepidopterologists. Entomol Heute 19:181–195
R Development Core Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Thomas JA (2007) Guide to butterflies of Britain and Ireland. Philip’s, London
Thompson JN (1988) Coevolution and alternative hypotheses on insect/plant interactions. Ecology 69:893–895
Thompson JN (2005) The geographic mosaic of coevolution. The University of Chigaco Press, Chicago
Thompson JN, Pellmyr O (1991) Evolution of oviposition behavior and host preference in Lepidoptera. Annu Rev Entomol 36:65–89
Verovnik R, Glogovčan P (2007) Morphological and molecular evidence of a possible hybrid zone of Leptidea sinapis and L. reali (Lepidoptera: Pieridae). Eur J Entomol 104:667–674
Via S (1999) Reproductive isolation between sympatric races of pea aphids. I. Gene flow restriction and habitat choice. Evolution 53:1446–1457
Vila R, Viader S, Jubany J (2003) Leptidea sinapis (Linnaeus, 1758) i L. reali (Reissinger 1988): dues ecpécies “bessones” a Catalunya i Andorra (Lepidoptera: Pieridae). Bull Soc Cat Lepid 90:25–47
Wedell N, Nylin S, Janz N (1997) Effects of larval host plant and sex on the propensity to enter diapause in the comma butterfly. Oikos 78:569–575
Wiklund C (1975) The evolutionary relationship between adult oviposition preferences and larval host plant range in Papilio machaon. Oecologia 18:85–197
Wiklund C (1977a) Courtship behaviour in relation to female monogamy in Leptidea sinapis (Lepidoptera). Oikos 29:275–283
Wiklund C (1977b) Oviposition, feeding and spatial separation of breeding and foraging habitats in a population of Leptidea sinapis (Lepidoptera). Oikos 28:56–68
Wiklund C (1982) Generalist versus specialist utilization of host plants among butterflies. In: Wisser JH, Minks AK (eds) Insect–plant relationships. PUDOC, Wageningen, pp 181–192
Wiklund C, Friberg M (2008) Enemy-free space and habitat-specific host specialisation in a butterfly. Oecologia 157:287–294
Wiklund C, Nylin S, Forsberg J (1991) Sex-related variation in growth-rate as a result of selection for large size and protandry in a bivoltine butterfly, Pieris napi. Oikos 60:241–250
Acknowledgements
We thank Martin Bergman and Martin Olofsson for assistance during the laboratory experiments, and for discussion and input on an earlier draft of this manuscript, and Helena Larsdotter Mellström and Didrik Vanhoenacker for useful comments on earlier drafts of this manuscript. This study complies with the current laws of Sweden and was financed by a grant from the Swedish Research Council to C.W.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Konrad Fiedler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Friberg, M., Wiklund, C. Host plant preference and performance of the sibling species of butterflies Leptidea sinapis and Leptidea reali: a test of the trade-off hypothesis for food specialisation. Oecologia 159, 127–137 (2009). https://doi.org/10.1007/s00442-008-1206-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1206-8