Abstract
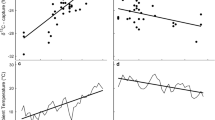
Omnivorous animals feed on several food items that often differ in macronutrient and isotopic composition. Macronutrients can be used for either metabolism or body tissue synthesis and, therefore, stable C isotope ratios of exhaled breath (δ13Cbreath) and tissue may differ. To study nutrient routing in omnivorous animals, we measured δ13Cbreath in 20-g Carollia perspicillata that either ate an isotopically homogeneous carbohydrate diet or an isotopically heterogenous protein-carbohydrate mixture. The δ13Cbreath converged to the δ13C of the ingested carbohydrates irrespective of whether proteins had been added or not. On average, δ13Cbreath was depleted in 13C by only ca. −2‰ in relation to the δ13C of the dietary carbohydrates and was enriched by +8.2‰ in relation to the dietary proteins, suggesting that C. perspicillata may have routed most ingested proteins to body synthesis and not to metabolism. We next compared the δ13Cbreath with that of wing tissue (δ13Ctissue) in 12 free-ranging, mostly omnivorous phyllostomid bat species. We predicted that species with a more insect biased diet—as indicated by the N isotope ratio in wing membrane tissue (δ15Ntissue)—should have higher δ13Ctissue than δ13Cbreath values, since we expected body tissue to stem mostly from insect proteins and exhaled CO2 to stem from the combustion of fruit carbohydrates. Accordingly, δ13Ctissue and δ13Cbreath should be more similar in species that feed predominantly on plant products. The species-specific differences between δ13Ctissue and δ13Cbreath increased with increasing δ15Ntissue, i.e. species with a plant-dominated diet had similar δ13Ctissue and δ13Cbreath values, whereas species feeding at a higher trophic level had higher δ13Ctissue than δ13Cbreath values. Our study shows that δ13Cbreath reflect the isotope ratio of ingested carbohydrates, whereas δ13C of body tissue reflect the isotope ratio of ingested proteins, namely insects, supporting the idea of isotopic routing in omnivorous animals.




Similar content being viewed by others
References
Adopo E, Péronnet F, Massicotte D, Brisson GR, Hillaire-Marvel C (1994) Respective oxidation of exogenous glucose and fructose given in the same drink during exercise. J Appl Physiol 76:1014–1019
Ambrose SH, Norr L (1993) Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate. In: Lambert JB, Grupe G (eds) Prehistoric human bone: Archeology at the molecular level. Springer, Berlin, pp 1–37
Ayliffe LK, Cerling TE, Robinson T, West A, Sponheimer M, Passey BH, Hammer J, Roeder B, Dearing MD, Ehleringer JR (2004) Turnover of carbon isotopes in tail hair and breath CO2 of horses fed an isotopically varied diet. Oecologia 139:11–22. doi:10.1007/s00442-003-1479-x
Carleton SA, Martinez del Rio C (2005) The effect of cold-induced increate metabolic rate on the rate of 13C and 15N in corporation in house sparrow (Passer domesticus). Oecologia 144:226–232
Carleton SA, Bakken BH, Martίnez del Rio C (2006) Metabolic substrate use and the turnover of endogenous energy reserves in broad-tailed hummingbirds (Selasphorus platycercus). J Exp Biol 209:2622–2627. doi:10.1242/jeb.02293
DeNiro MJ, Epstein S (1977) Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197:261–263. doi:10.1126/science.327543
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506. doi:10.1016/0016-7037(78)90199-0
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351. doi:10.1016/0016-7037(81)90244-1
Fry B (2006) Stable isotope ecology. Springer, Berlin
Gardner AL (1976) Feeding habits. In: Baker RJ, Jones JK Jr, Carter DC (eds) Biology of the new world family Phyllostomidae. II. Special publications. The Museum, Texas Tech Univeristy, Texas
Hatch KA, Pinshow B, Speakman JR (2002a) Carbon isotope ratios in exhaled CO2 can be used to determine not just present, but also past diets in birds. J Comp Physiol B 172:263–268. doi:10.1007/s00360-002-0251-6
Hatch KA, Pinshow B, Speakman JR (2002b) The analysis of 13C/12C ratios in exhaled CO2: its advantages and potential application to field research to infer diet, changes in diet over time, and substrate metabolism in birds. Integr Comp Biol 42:21–33. doi:10.1093/icb/42.1.21
Herrera LG, Fleming TH, Sternberg LS (1998) Trophic relationships in a neotropical bat community: a preliminary study using carbon and nitrogen isotopic signatures. Trop Ecol 39:23–29
Herrera LG, Hobson KA, Mirón L M, Ramírez P N, Mendéz C G, Sánchez-Cordero V (2001) Source of protein in two species of phytophagous bats in a seasonal dry forest: evidence from stable-isotope analysis. J Mammal 82:352–361. doi:10.1644/1545-1542(2001)082<0352:SOPITS>2.0.CO;2
Herrera LG, Gutierrez EC, Hobson KA, Altube MB, Díaz WGC, Sánchez-Cordero V (2002) Sources of assimilated protein in five species of New World frugivorous bats. Oecologia 133:280–287. doi:10.1007/s00442-002-1036-z
Martínez del Río C, Wolf BO (2005) Mass-balance models for animal isotopic ecology. In: Stark JM, Wang T (eds) Physiological and ecological adaptations to feeding in vertebrates. Science Publishers, Enfield, pp 141–174
Perkins SE, Speakman JR (2001) Measuring natural abundance of 13C in respired CO2: variability and implications for non-invasive dietary analysis. Funct Ecol 15:791–797. doi:10.1046/j.0269-8463.2001.00577.x
Péronnet F (2003) Oxydation des glucides ingérés pendant exercice. Actual Chim 8(9):33–37
Podlesak DW, McWilliams SR, Hatch KA (2005) Stable isotopes in breath, blood, feces and feathers can indicate intra-individual changes in the diet of migratory songbirds. Oecologia 142:501–510. doi:10.1007/s00442-004-1737-6
Podlesak DW, McWilliams SR (2006) Metabolic routing of dietary nutrients in birds: Effect of diet quality and macronutrient composition revealed using stable isotopes. Phys Biochem Zool 79:534–549
Saito M, Murakami E, Suda M (1976) Circadian rhythms in disaccharidases of rat small intestine and its relation to food intake. Biochim Biophys Acta 421:177–179
Schondube J, Martínez del Rio C, Herrera LG (2001) Diet and the evolution of digestion and renal function in phyllostomid bats. Zoology 104:59–74
Schwarcz HP, Schoeninger MJ (1991) Stable isotope analyses in human nutritional ecology. Yearb Phys Anthropol 34:283–321
Sponheimer M, Robinson TF, Cerling TE, Tegland L, Roeder BK, Ayliffe L, Dearing MD, Ehleringer JR (2006) Turnover of stable carbon isotopes in the muscle, liver, and breath CO2 of alpacas (Lama pacos). Rapid Commun Mass Spectrom 20:1395–1399
Stevenson NR, Ferrigni F, Parnicky K, Day S, Fierstein JS (1975) Effects of changes in feeding schedule on the diurnal rhythms and daily activity levels of intestinal brush border enzymes and transport systems. Biochim Biophys Acta 406:131–145
Tieszen RM, Boutton TW, Tesdahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57:32–37. doi:10.1007/BF00379558
Tieszen LL, Fagre T (1993) Effect of diet quality and composition on the isotopic composition of respiratory CO2, bone collagen, bioapatite, and soft tissues. In: Lambert JB, Grupe G (eds) Prehistoric human bone archaeology at the molecular level. Springer, Berlin, Heidelberg, New York, pp 121-155
Timm RM, LaVal RK (1998) A field key to the bats of Costa Rica. Occas Publ Ser Univ Kan 22:1–30
Voigt CC, Matt F (2004) Nitrogen stress causes unpredictable enrichments of 15 N in two nectar-feeding bat species. J Exp Biol 207:1741–1748. doi:10.1242/jeb.00929
Voigt CC, Speakman JR (2007) A mammal nectar specialist fuels its metabolically expensive nocturnal life directly and almost exclusively with exogenous carbohydrates. Funct Ecol 21:913–921. doi:10.1111/j.1365-2435.2007.01321.x
Voigt CC, Matt F, Michener R, Kunz TH (2003) Low rates of carbon isotope turnover in tissues of two nectar-feeding bat species (Glossophaginae, Phyllostomidae). J Exp Biol 206:1419–1427. doi:10.1242/jeb.00274
Voigt CC, Lehmann GUC, Michener R, Joachimski MM (2006) Nuptial feeding is reflected in tissue nitrogen isotope ratios of female katydids. Funct Ecol 20:656–661. doi:10.1111/j.1365-2435.2006.01154.x
Voigt CC, Dechmann DKN, Bender J, Rinehart JB, Michener RH, Kunz TH (2007) Mineral licks attract neotropical seed-dispersing bats. Res Lett Ecol, Article ID 34212 doi:10.1155/2007/34212
Voigt CC, Grasse P, Rex K, Hetz SK, Speakman JR (2008) Bat breath reveals metabolic substrate use in free-ranging vampires. J Comp Physiol B 178:9–16. doi:10.1007/s00360-007-0194-z
Welch KC Jr, Bakken BH, Martίnez del Rio B, Suarez RK (2006) Hummingbirds fuel hovering flight with newly ingested sugar. Physiol Biochem Zool 79:1082–1087
Wendeln MC, Runkle JR, Kalko EKV (2000) Nutritional values of 14 fig species and bat feeding preferences in Panama. Biotropica 32:489–501
Acknowledgements
Peter Thompson and Dr Paula Redman analysed the samples at the Aberdeen Centre for Energy Regulation and Obesity. We thank Carolin Werres for help during the field work at La Selva Biological Station and OTS for providing support and infrastructure during the course of this study. We acknowledge the generous support of the Costa Rican authorities, especially Javier Guevara at SINAC. The experiments complied with the current laws of Costa Rica. We thank Dr Detlev Kelm for commenting on an earlier version of this manuscript. This work was financed by a grant from the Deutsche Forschungsgemeinschaft to CCV (Vo890/7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Carlos Martinez del Rio.
Rights and permissions
About this article
Cite this article
Voigt, C.C., Rex, K., Michener, R.H. et al. Nutrient routing in omnivorous animals tracked by stable carbon isotopes in tissue and exhaled breath. Oecologia 157, 31–40 (2008). https://doi.org/10.1007/s00442-008-1057-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1057-3