Abstract
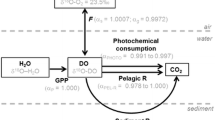
Dissolved O2 is an important aquatic ecosystem health indicator. Metabolic and gas exchange (G) rates, which control O2 concentration, are affected by nutrient loading and other environmental factors. Traditionally, aquatic metabolism has been reported as primary production:community respiration (P:R) ratios using diel measurements and interpretations of dissolved O2 and/or CO2 concentrations, and recently using stable isotopes (δ18O, Δ17O) and steady state assumptions. Aquatic ecosystems, such as rivers and ponds, are not at steady state and exhibit diel changes, so steady state approaches are often inappropriate. A dynamic O2 stable isotope model (photosynthesis–respiration–gas exchange; PoRGy) is presented here, requiring a minimum of parameters to quantify daily averaged P, R, and G rates under transient field conditions. Unlike steady state approaches, PoRGy can address scenarios with 100% O2 saturation but with δ18O-O2 values that are not at air equilibrium. PoRGy successfully accounts for isotopic G when applied to an oxygen isotope equilibration laboratory experiment. PoRGy model results closely matched the diel O2 and δ18O-O2 data from three field sites with different P:R:G ratios and various P, R and G rates. PoRGy provides a new research tool to assess ecosystem health and to pose environmental impact-driven questions. Using daily averaged rates was successful and thus they can be used to compare ecosystems across seasons and landscapes.





Similar content being viewed by others
Notes
The 18O:16O ratio of O2 is measured and reported as the parts per thousand deviation from Vienna standard mean ocean water (VSMOW): \( \delta = {\left( {\frac{{R_{{\rm sample}} }} {{R_{{\rm VSMOW}} }} - 1} \right)}\) where R is the 18O:16O ratio. Isotope fractionation factors for different processes are denoted as α values: \( \alpha = \frac{{R_{\rm b} }} {{R_{\rm a} }} \) where R is the 18O:16O ratio of the reactant (a) and product (b).
References
Andrews SS, Caron S, Zafiriou OC (2000) Photochemical oxygen consumption in marine waters: a major sink for colored dissolved organic matter? Limnol Oceanogr 45:267–277
Barkan E, Luz B (2003) Measurements of 17O/16O and 18O/16O of O2 and O2/Ar ratio in air. Rapid Commun Mass Spec 17:2809–2814 doi:10.1002/rcm.1267
Barth JAC, Tait A, Bolshaw M (2004) Automated analyses of 18O/16O ratios in dissolved oxygen from 12-mL water samples. Limnol Oceanogr Meth 2:35–41
Bender ML, Sowers T, Labeyrie L (1994) The Dole effect and its variations during the last 130,000 years as measured in the Vostok ice core. Global Biogeochem Cycles 8:363–376
Bennoun P (2002) The present model for chlororespiration. Photosyn Res 73:273–277
Benson BB, Krause D Jr, Peterson MA (1979) The solubility and isotopic fractionation of gases in dilute aquatic solution. I. Oxygen. J Solution Chem 8:655–690
Bird RE, Hulstrom RL (1981) A simplified clear sky model for direct and diffuse insolation on horizontal surfaces. SERI/TR-642–761. Solar Energy Research Institute, Golden
Bodaly RA, Beaty KG, Hendzel LH, Majewski AR, Paterson MJ, Rolfhus KR, Penn AF, St.Louis VL, Hall BD, Matthews CJD, Cherewyk KA, Mailman M, Hurley JP, Schiff SL, Venkiteswaran JJ (2004) The use of experimental reservoirs to explore the mercury and greenhouse gas impacts of hydro-electric developments: the FLUDEX experiment. Environ Sci Technol 38:337A–352A
Borsuk ME, Stow CA, Reckhow KH (2004) Confounding effect of flow on estuarine response to nitrogen loading. Environ Eng 130:605–614 doi:10.1061/(ASCE)0733-9372(2004)130:6(605)
Bowie GL, Mills WB, Porcella DB, Campbell CL, Pagenkopf JR, Rupp GL, Johnson KM, Chan PWH, Gherini SA, Chamberlin CE (1985) Rates, constants, and kinetic formulations in surface water quality modelling, 2nd edn. EPA/600/3-85/040, USEPA, Athens
Brandes JA, Devol AH (1997) Isotopic fractionation of oxygen and nitrogen in coastal marine sediments. Geochim Cosmochim Acta 61:1793–1801
Broecker WS (1985) How to build a habitable planet. Eldigio, Palisades
Chapra SC, Di Toro DM (1991) Delta method for estimating primary production, respiration, and reaeration in streams. J Environ Eng ASCE 117:640–655
Carignan R, Planas D, Vis C (2000) Planktonic production and respiration in oligotrophic Shield lakes. Limnol Oceanogr 45:189–199
Churchill MA, Elmore HL, Buckingham RA (1962) The prediction of stream reaeration rates. J Environ Eng ASCE 88(SA4):1–46
Coplen TB, Hopple JA, Böhlke JK, Peiser HS, Rieder SE, Krouse HR, Rosman KJR, Ding T, Vocke RD Jr, Révész KM, Lamberty A, Taylor P, De Bièvre P (2001) Compilation of minimum and maximum isotope ratios of selected elements in naturally occurring terrestrial materials and reagents. US Geological Survey Water-Resources Investigations report 01-4222. USGS
Crusius J, Wanninkhof R (2003) Gas transfer velocities measured at low wind speed over a lake. Limnol Oceanogr 48:101–1017
del Giorgio PA, Peters DH (1994) Patterns in planktonic P:R ratios in lakes: influence of lake trophy and dissolved organic carbon. Limnol Oceanogr 39:772–787
Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Blackwell, Malden
Fry B (2006) Stable isotope ecology. Springer, Berlin Heidelberg New York
Gelda RK, Auer MT, Effler SW, Chapra SC, Storey ML (1996) Determination of reaeration coefficients: whole-lake approach. J Environ Eng ASCE 122:269–275. doi:10.1061/(ASCE)0733-9372(1996)122:4(269)
Guy RD, Berry JA, Fogel ML, Hoering TC (1989) Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta 177:483–491
Guy RD, Fogel ML, Berry JA (1993) Photosynthetic fractionation of the stable isotopes of oxygen and carbon. Plant Physiol 101:37–47
Hartnett H, Devol A, Brandes J, Chang B (2005) Oxygen isotope fractionation in marine sediments during respiration. Geochim Cosmochim Acta 69:A579
Helman Y, Barkan E, Eisenstadt D, Luz B, Kaplan A (2005) Fractionation of the three stable oxygen isotopes by oxygen-producing and oxygen-consuming reactions in photosynthetic organisms. Plant Physiol 138:2292–2298. doi:10.1104/pp.105.063768
Hendricks MB, Bender ML, Barnett BA, Strutton P, Chavez FP (2005) Triple isotope composition of dissolved O2 in the equatorial Pacific: a tracer of mixing, production, and respiration. J Geophys Res 110:C12021 doi:10.1029/2004JC002735
Hendry MJ, Wassenaar LI, Birkham TK (2002) Microbial respiration and diffusive transport of O2, 16O2, and 18O16O in unsaturated soils: a mesocosm experiment. Geochim Cosmochim Acta 66:3367–3374. doi:10.1016/S0016-7037(02)00949-3
Hoffmann G, Cuntz M, Weber C, Ciais P, Friedlingstein P, Heinmann M, Jouzel J, Kaduk J, Maier-Reimer E, Seibt U, Six K (2004) A model of the Earth’s Dole effect. Global Biogeochem Cycles 18:GB1008. doi:10.1029/2003GB002059
Horita J, Kendall C (2004) Stable isotope analysis of water and aqueous solutions by conventional dual-inlet mass spectrometry. In: de Groot PA (ed) Handbook of stable isotope analytical techniques, vol 1. Elsevier, Amsterdam, pp 1–37
Hornberger GM, Kelly MG (1975) Atmospheric reaeration in a river using productivity analysis. J Environ Eng ASCE 101:729–739
Jha R, Ojha CSP, Bhatia KSS (2001) Refinement of predictive reaeration equations for a typical Indian river. Hydrol Proc 15:1047–1060. doi:10.1002/hyp.177
Jha R, Ojha CSP, Bhatia KSS (2004) A supplementary approach for estimating reaeration rate coefficients. Hydrol Proc 18:65–79. doi:10.1002/hyp.1312
Juranek LW, Quay PD (2005) In vitro and in situ gross primary and net community production in the North Pacific Subtropical Gyre using labelled and natural abundance isotopes of dissolved O2. Global Bioeochemical Cycles 19:GB3009. doi:10.1029/2004GB002384
Kiddon J, BenderML, Orchardo J, Caron DA, Goldman JC, Dennett M (1993) Isotopic fractionation of oxygen by respiring marine organisms. Global Biogeochem Cycles 7:679–694
Kirschbaum MUF (1995) The temperature-dependence of soil organic-matter decomposition, and the effect of global warming on soil organic-C storage. Soil Biol Biochem 27:753–760
Knox M, Quay PD, Wilbur D (1992) Kinetic isotopic fractionation during air–water gas transfer of O2, N2, CH4, and H2. J Geophys Res 97:20335–20343
Konikow LF, Bredehoeft JD (1992) Groundwater models cannot be validated. Adv Wat Res 15:75–83
Kroopnick P, Craig H (1972) Atmospheric oxygen: Isotopic composition and solubility fractionation. Science 175:54–55
Langbein WB, Durum WH (1967) The aeration capacity of streams. USGS circular no. 542. USGS, Washington, D.C.
Laws EA, Landry MR, Barber RT, Campbell L, Dickson ML, Marra J (2000) Carbon cycling in primary production bottle incubations: inferences from grazing experiments and photosynthetic studies using 14C and 18O in the Arabian Sea. Deep-Sea Res II 47:1339–1352. doi:10.1016/S0967-0645(99)00146-0
Luz B, Barkan E (2000) Productivity with the triple-isotope composition of dissolved oxygen. Science 288:2028–2031
Luz B, Barkan E, Bender ML, Thiemens MH, Boering KA (1999) Triple-isotope composition of atmospheric oxygen as a tracer of biosphere productivity. Nature 400:547–550. doi:10.1038/22987
Marzolf ER, Mulholland PJ, Steinman AD (1994) Improvements to the diurnal upstream–downstream dissolved-oxygen change technique for determining whole-stream metabolism in small streams. Can J Fish Aquat Sci 51:1591–1599
McBride GB (2002) Calculating stream reaeration coefficients from oxygen profiles. J Environ Eng ASCE 128:384–386. doi:10.1061/(ASCE)0733-9372(2002)128:4(384)
Miles CJ, Brezonik PL (1981) Oxygen consumption in humic-colored waters by a photochemical ferrous-ferric catalytic cycle. Environ Sci Technol 15:1089–095. doi:10.1021/es00091a010
Moog DB, Jirka GH (1998) Analysis of reaeration equations using mean multiplicative error. J Environ Eng ASCE 124:104–110. doi:10.1061/(ASCE)0733-9372(1998)124:2(104)
Mulholland PJ, Houser JN, Maloney KO (2005) Stream diurnal dissolved oxygen profiles as indicators of in-stream metabolism and disturbance effects: Fort Benning as a case study. Ecol Indicat 5:243–252. doi:10.1016/j.ecolind.2005.03.004
O’Connor DJ, Dobbins WE (1958) Mechanism of reaeration in natural streams. Am Soc Civ Eng Trans 123:641–684
Odum HT (1956) Primary production in flowing waters. Limnol Oceanogr 1:102–117
Oreskes N, Belitz K, Shraderfrechette K (1994a) The meaning of models—response. Science 264:331–331
Oreskes N, Shraderfrechette K, Belitz K (1994b) Verification, validation, and confirmation of numerical-models in the earth-sciences. Science 263:641–646
Osmond CB (1981) Photo-respiration and photoinhibition some implications for the energetics of photosynthesis. Biochim Biophys Acta 639:77–98
Owens M, Edwards RW, Gibbs JW (1964) Some reaeration studies in streams. Air Water Pollut 35:469–486
Pace ML, Prairie YT (2005) Respiration in lakes. In: del Giorgio PA, Williams PJ le B (eds) Respiration in aquatic ecosystems. Oxford University Press, Oxford, pp 103–121
Parker SR, Poulson SR, Gammons CH, Degrandpre MD (2005) Biogeochemical controls on diel cycling of stable isotopes of dissolved O2 and dissolved inorganic carbon in the Big Hole River, Montana. Environ Sci Technol 39:7134–7140. doi:10.1021/es0505595
Prairie YT, Bird DF, Cole JC (2002) The summer metabolic balance in the epilimnion of southeastern Quebec lakes. Limnol Oceanogr 47:316–321
Quay PD, Emerson S, Wilbur DO, Stump C (1993) The δ18O of dissolved O2 in the surface waters of the sub-arctic pacific: a tracer of biological productivity. J Geophys Res 98:8447–8458
Quay PD, Wilbur DO, Richey JE, Devol AH (1995) The 18O:16O of dissolved oxygen in rivers and the lakes in the Amazon Basin: determining the ratio of respiration to photosynthesis rates in freshwater. Limnol Oceanogr 40:718–729
Russ ME, Ostrom NE, Gandhi H, Ostrom PH, Urban NR (2004) Temporal and spatial variations in R:P ratios in Lake Superior, an oligotrophic freshwater environment. J Geophys Res 109:C10S12. doi:10.1029/2003JC001890
Sarma VVSS, Abe O, Hashimoto S, Hinuma A, Saino T (2005) Seasonal variations in triple oxygen isotopes and gross oxygen production in the Sagami Bay, central Japan. Limnol Oceanogr 50:544–552
Sarma VVSS, Abe O, Hinuma A, Saino T (2006) Short-term variation of triple oxygen and gross oxygen production in the Sagami Bay, central Japan. Limnol Oceanogr 51:1432–1442
Schindler DW (1987) Detecting ecosystem responses to anthropogenic stress. Can J Fish Aquat Sci 44[Suppl 1]:6–25
Stevens CLR, Schultz D, Vanbaalen C, Parker PL (1975) Oxygen isotope fractionation during photosynthesis in a blue-green and a green-alga. Plant Physiol 56:126–129
Stumm W, Morgan JJ (1996) Aquatic chemistry, 3rd edn. Wiley–Interscience, New York
Taylor BE, Wheeler MC, Nordstrom DK (1984) Stable isotope geochemistry of acid-mine drainage—experimental oxidation of pyrite. Geochim Cosmochim Acta 48:2669–2678
van Dam O (2001) Forest filled with gaps. Effects of gap size on water and nutrient cycling in tropical rain forest. A study in Guyana. PhD thesis, Utrecht University, Utrecht
Venkiteswaran JJ, Schiff SL, Wassenaar LI (2007) Aquatic metabolism and ecosystem health assessment using dissolved O2 stable isotope diel curves. Ecol Appl (Unpublished)
Wang XF, Veizer J (2000) Respiration-photosynthesis balance of terrestrial aquatic ecosystems, Ottawa area, Canada. Geochim Cosmochim Acta 64:3775–3786. doi:10.1016/S0016-7037(00)00477-4
Wang XF, Veizer J (2004) Erratum to Xuefeng Wang and Jan Veizer (2000), respiration–photosynthesis balance of terrestrial aquatic ecosystems, Ottawa area, Canada. Geochim Cosmochim Acta 64(22), 3775–3786. Geochim Cosmochim Acta 68:933–944. doi:10.1016/S0016-7037(03)00490-3
Wassenaar LI, Koehler G (1999) An on-line technique for the determination of the δ18O and δ17O of gaseous and dissolved oxygen. Anal Chem 71:4965–4968
Weiss RF (1970) Solubility of nitrogen, oxygen and argon in water and seawater. Deep-Sea Res 17:721–735
Wetzel R (2001) Limnology—lake and river ecosystems. Academic Press, San Diego, Calif
Wilcock RJ, Nagels JW, McBride GB, Collier KJ, Wilson BT, Huser BA (1998) Characterisation of lowland streams using a single-station diurnal curve analysis model with continuous monitoring data for dissolved oxygen and temperature. N Z J Mar Fresh Res 32:67–79
Woo MK, Roswell RD (1993) Hydrology of a prairie slough. J Hydrol 146:175–207
Yan ND (2005) Research needs for the management of water quality issues, particularly phosphorus and oxygen concentrations, related to salmonid cage aquaculture in Canadian freshwaters. Environ Rev 13:1–9
Acknowledgements
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic grant (S. L. S. and L. I. W.), Environment Canada (L. I. W.), the Canadian Foundation for Climate and Atmospheric Sciences (CFCAS) (S. L. S.), an Ontario Graduate Scholarship (J. J. V.), and Environment Canada’s Science Horizons Youth Internship Program (S. L. S.). Analytical development at the University of Waterloo was funded by an NSERC Discovery grant (S. L. S.), CFCAS (S. L. S.), and the Centre for Research in Earth and Space Technology (S. L. S.). Andrea Wojtyniak, Kevin Maurice, and Matthijs Vlaar provided field assistance. Richard Elgood, Geoff Koehler, and Daryl Halliwell provided additional field and laboratory assistance. FLUDEX was funded by Fisheries and Oceans Canada, Manitoba Hydro, and Hydro-Québec.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dan Yakir.
Rights and permissions
About this article
Cite this article
Venkiteswaran, J.J., Wassenaar, L.I. & Schiff, S.L. Dynamics of dissolved oxygen isotopic ratios: a transient model to quantify primary production, community respiration, and air–water exchange in aquatic ecosystems. Oecologia 153, 385–398 (2007). https://doi.org/10.1007/s00442-007-0744-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0744-9