Abstract
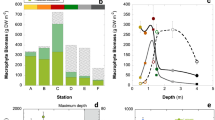
Photosynthesis–irradiance relationships of macroalgal communities and thalli of dominant species in shallow coastal Danish waters were measured over a full year to test how well community production can be predicted from environmental (incident irradiance and temperature) and community variables (canopy absorptance, species number and thallus metabolism). Detached thalli of dominant species performed optimally at different times of the year, but showed no general seasonal changes in photosynthetic features. Production capacity of communities at high light varied only 1.8-fold over the year and was unrelated to incident irradiance, temperature and mean thallus photosynthesis, while community absorptance was a highly significant predictor. Actual rates of community photosynthesis were closely related to incident and absorbed irradiance alone. Community absorptance in turn was correlated to canopy height and species richness. The close relationship of community photosynthesis to irradiance is due to the fact that (1) large differences in thallus photosynthesis of individual species are averaged out in communities composed of several species, (2) seasonal replacement of species keeps communities metabolically active, and (3) maximum possible absorptance at 100% constrains the total photosynthesis of all species. Our results imply that the photosynthetic production of macroalgal communities is more predictable than their complex and dynamic nature suggest and that predictions are possible over wide spatial scales in coastal waters by measurements of vegetation cover, incoming irradiance and canopy absorptance.








Similar content being viewed by others
References
Begon M, Harper JL, Townsend CR (1996) Ecology: Individuals, populations and communities, 3rd edn. Blackwell Science, Oxford, pp 1068
Behrenfeld MJ, Prasil O, Babin M, Bruyant F (2004) In search of a physiological basis for covariations in light-limited and light-saturated photosynthesis. J Phycol 40:4–25
Beyschlag W, Ryel R (1998) Modelling leaf/canopy production. In: Raghavendra AS (ed) Photosynthesis, a comprehensive treatise. Cambridge University Press, Cambridge, pp 305–319
Binzer T, Middelboe AL (2005) From thallus to communities: scale effects and photosynthetic performance in macroalgae communities. Mar Ecol Prog Ser 287:65–75
Binzer T, Sand-Jensen K (2002a) Importance of structure and density of macroalgae communities (Fucus serratus) for photosynthetic production and light utilisation. Mar Ecol Prog Ser 235:53–62
Binzer T, Sand-Jensen K (2002b) Production in aquatic macrophyte communities: a theoretical and empirical study of the influence of spatial light distribution. Limnol Oceanogr 47:1742–1750
Borum J, Sand-Jensen K (1996) Is total primary production in shallow coastal marine waters stimulated by nitrogen loading? Oikos 76:406–410
Brenchley JL, Raven JA, Johnston AM (1997) Resource acquisition in two intertidal fucoid seaweeds, Fucus serratus and Himanthalia elongata: seasonal variation and effects of reproductive development. Mar Biol 129:367–375
Cade BS, Terrell JW, Schroeder RL (1999) Estimating effects of limiting factors with regression quantiles. Ecology 80:311–323
Carpenter RC (1985) Relationships between primary production and irradiance in coral-reef algal communities. Limnol Oceanogr 30:784–793
Cheshire AC, Westphalen G, Wenden A, Scriven LJ, Rowland BC (1996) Photosynthesis and respiration of phaeophycean-dominated macroalgal communities in summer and winter. Aquat Bot 55:159–170
Davison IR, Greene RM, Podolak EJ (1991) Temperature–acclimation of respiration and photosynthesis in the brown alga Laminaria saccharina. Mar Biol 110:449–454
Doak DF, Bigger D, Harding EK, Marvier MA, O’Malley RE, Thomson D (1998) The statistical inevitability of stability-diversity relationships in community ecology. Am Nat 151:264–276
Enriquez S, Duarte CM, Sand-Jensen K (1995) Patterns in the photosynthetic metabolism of Mediterranean macrophytes. Mar Ecol Prog Ser 119:243–252
Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Blackwell, Malden, MA
Frost-Christensen H, Sand-Jensen K (1992) The quantum efficiency of photosynthesis in macroalgae and submerged angiosperms. Oecologia 91:377–384
Gunnarsson K, Ingolfsson A (1995a) Seasonal changes in the abundance of intertidal algae in Southwestern Iceland. Bot Mar 38:69–77
Gunnarsson K, Ingolfsson A (1995b) Seasonal-changes in the abundance of intertidal algae in Southwestern Iceland. Bot Mar 38:69–77
Hein M, Hansen JB, Ditlevsen GH, Nielsen JB, Rasmussen J, Sørensen K, Angantyr LA (2004) Overvågning af Øresund 2003. Frederiksborg Amt, Københavns Amt, Roskilde Amt and Københavns Kommunes Miljøkontrol, Denmark
Jørgensen EG (1969) The adaptation of plankton algae. IV Light adaptation in different algal species. Physiol Plant Pathol 22:1307–1315
King RJ, Schramm W (1976a) Photosynthetic rates of benthic marine algae in relation to light intensity and seasonal variations. Mar Biol 37:215–222
King RJ, Schramm W (1976b) Photosynthetic rates of benthic marine-algae in relation to light-intensity and seasonal-variations. Mar Biol 37:215–222
Kirk JTO (1994) Light and photosynthesis in aquatic ecosystems. Cambridge University Press, Cambridge
Lederman TC, Tett P (1981) Problems in modelling the photosynthesis—light relationship for phytoplankton. Bot Mar 43:305–314
Lirman D, Biber P (2000) Seasonal dynamics of macroalgal communities of the northern Florida reef tract. Bot Mar 43:305–314
Mann KH (1973) Seaweeds—their productivity and strategy for growth. Science 182:975–981
Mann KH (2000) Ecology of coastal waters. With implications for management. Blackwell, Oxford
Markager S, Sand-Jensen K (1994) The physiology and ecology og light-growth relationship in macroalgae. Prog Phycol Res 10:209–298
Markager S, Sand-Jensen K (1996) Implications of thallus thickness for growth irradiance relationships of marine macroalgae. Eur J Phycol 31:79–87
Middelboe AL, Binzer T (2004) Importance of canopy structure on photosynthesis in single- and multi-species assemblages of marine macroalgae. Oikos 107:422–432
Revsbech NP (1989) An oxygen microsensor with a guard cathode. Limnol Oceanogr 34:474–478
Ruimy a, Jarvis PG, Baldocchi DD, Saugier B (1995) CO2 fluxes over plant canopies and solar radiation: a review. Adv Ecol Res 26:1–68
Sand-Jensen K (1988) Photosynthetic responses of Ulva lactuca at low light. Mar Ecol Prog Ser 50:195–201
Thom RM, Albright RG (1990) Dynamics of benthic vegetation standing stock, irradiance, and water properties in central Puget Sound. Mar Biol 104:129–141
Tilman D (1999) The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80:1455–1474
Wallentinus I (1977) Productivity studies on Baltic macroalgae. J Phycol 13:71
Weykam G, Wiencke C (1996) Seasonal photosynthetic performance of the endemic Antarctic red alga Palmaria decipiens (Reinsch) Ricker. Polar Biol 16:357–361
Williams GA (1996) Seasonal variation in a low shore Fucus serratus (Fucales, Phaeophyta) population and its epiphytic fauna. Hydrobiologia 327:191–197
Acknowledgment
This work was supported by grants from the Carlsberg Foundation to ALM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christian Koerner.
Rights and permissions
About this article
Cite this article
Middelboe, A.L., Sand-Jensen, K. & Binzer, T. Highly predictable photosynthetic production in natural macroalgal communities from incoming and absorbed light. Oecologia 150, 464–476 (2006). https://doi.org/10.1007/s00442-006-0526-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0526-9