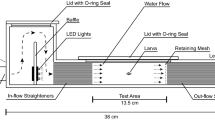
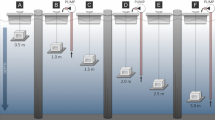
Abstract
While known to be uniformly non-feeding, short-lived, and potentially short dispersing, sponge larvae display different behaviours (swimming ability and taxis). Our aim was to show whether sponge larvae with different behaviours exhibit different dispersal strategies under variable intensity of water movements. We first assessed the distribution of larvae of six taxa: Dictyoceratida spp., Dysidea avara, Crambe crambe, Phorbas tenacior, Scopalina lophyropoda, and Cliona viridis, collected through plankton sampling, and the abundance of the corresponding adult sponges across three hard bottom communities and a sandy bottom from a north-west Mediterranean rocky shore. We then tested adult–larvae couplings (abundance of larvae vs abundance of adults) under increasing levels of water movements (surge) to assess the importance of this environmental factor in driving differences in dispersal strategies. Adults of Dictyoceratida spp., D. avara, and P. tenacior were most abundant in semi-dark caves (SDC), C. crambe and C. viridis in communities of sciaphilic algae (SA), whereas the distribution of S. lophyropoda was extremely patchy, being present almost only in the SA community of one of the five stations studied. Larvae of Dictyoceratida spp. and P. tenacior were more abundant in the SDC, whereas D. avara and C. crambe were homogeneously distributed across the communities. The larvae of C. viridis were more abundant in the SA communities and the S. lophyropoda larvae were mostly present in one station and one community (SA). Increased water movement did not modify the adult–larvae coupling for Dictyoceratida spp., D. avara, and C. crambe, whereas it broke up the positive association for P. tenacior and to some extent S. lophyropoda. For C. viridis, possible variability in adult–larvae coupling was not tested because the larvae were collected on only one day under calm sea conditions. We confirm that efficient-swimming larvae with some cue response can actively counteract hydrodynamic forces and highlight the importance of both larval behaviour and environmental conditions in determining small-scale patterns of dispersal.


Similar content being viewed by others
References
Abelson A, Denny M (1997) Settlement of marine organisms in flow. Annu Rev Ecol Syst 28:317–339
Amano S (1986) Larval release in response to a light signal by the intertidal sponge Halichondria panicea. Biol Bull 171:371–378
Amano S (1988) Morning release of larvae controlled by the light in an intertidal sponge, Callyspongia ramosa. Biol Bull 175:181–184
Becerro MA, Uriz MJ, Turon X (1994) Trends in space occupation by the encrusting sponge Crambe crambe: variation in shape as a function of size and environment. Mar Biol 121:301–307
Bergquist PR, Sinclair ME (1968) The morphology and behaviour of larvae of some intertidal sponges. NZ J Mar Freshw Res 2:426–437
Bergquist PR, Sinclair ME, Hogg JJ (1970) Adaptation to intertidal existence: reproductive cycles and larval behaviour in Demospongiae. Symp Zool Soc Lond 25:247–271
Boury-Esnault N, Rützler K (1997) Thesaurus of sponge morphology. Smith Contr Zool 596:1–55
Burdett-Coutts V, Metaxas A (2004) The effect of the quality of food patches on vertical distribution of the sea urchins Lytechinus variegatus (Lamark) and Strongylocentrotus droebachensis (Mueller). J Exp Mar Biol Ecol 308:221–236
Fisher R (2005) Swimming speeds of larval coral reef fishes: impacts of self-recruitment and dispersal. Mar Ecol Prog Ser 285:223–232
Gilg MR, Hilbish TJ (2003) The geography of marine larval dispersal: coupling genetics with fine-scale physical oceanography. Ecology 84:2989–2998
Graham KR, Sebens KP (1996) The distribution of marine invertebrate larvae near vertical surfaces in the rocky subtidal zone. Ecology 77:933–949
Grosberg RK (1987) Limited dispersal and proximity-dependent mating success in the colonial ascidian Botryllus shlosseri. Evolution 41:372–384
Jackson JBC (1985) Distribution and ecology of clonal and aclonal benthic invertebrates. In: Jackson JBC, Buss LW, Cook E (eds) Population biology and evolution of clonal organisms. Yale University, New Haven, pp 297–356
Keough MJ (1986) The distribution of a bryozoan on seagrass blades: settlement, growth, and mortality. Ecology 67:846–857
Keough MJ, Black KP (1996) Predicting the scale of marine impacts: Understanding planktonic links between population. In: Schmitt RJ, Osenberg CW (eds) The design of ecological impact studies: conceptual issues and application in coastal marine habitats. Academic, Orlando, pp 199–234
Leis JM, Sweatman HPA, Reader SE (1996) What pelagic stages of coral reef fishes are doing in the blue water: daytime field observations of larval behavioural capabilities. Mar Freshw Res 47:401–411
Levin LA, Huggett D, Myers P, Bridges T, Weaver J (1993) Rare-earth tagging methods for the study of larval dispersal by marine invertebrates. Limnol Oceanogr 38:346-360
Leys SP, Degnan BM (2001) Cytological basis of photoresponsive behavior in a sponge larva. Biol Bull 201:323–338
Lindquist N, Hay ME (1996) Palatability and chemical defence of marine invertebrate larvae. Ecol Monogr 62:547–568
Linquist N, Bolser RC, Laing K (1997) Timing of larval release by two Caribbean demosponges. Mar Ecol Prog Ser 155:309–316
Maldonado M, Bergquist PR (2002) Phylum porifera. In: Young CM (ed) Atlas of marine invertebrate larvae. Academic, London, pp 21–50
Maldonado M, Uriz MJ (1998) Microrefuge explotation by subtidal encrusting sponges: patterns of settlement and post-settlement survival. Mar Ecol Prog Ser 174:141–150
Maldonado M, Young CM (1996) Effects of physical factors on larval behavior, settlement and recruitment of four tropical demosponges. Mar Ecol Prog Ser 138:169–180
Maldonado M, Durfort M, McCarty DA, Young CM (2003) The cellular basis of photobehavior in the tufted parenchymella larva of demosponges. Mar Biol 143:427–441
Mariani S (2002) Larval supply and recruitment of invertebrates in the western Mediterranean: patterns in contrasting benthic communities. PhD thesis, University of Barcelona
Mariani S (2003) Recruitment in invertebrates with short-lived larvae: the case of the bryozoan Disporella hispida (Fleming). Helgol Mar Res 57:47–53
Mariani S, Uriz MJ, Turon X (2000) Larval bloom of the oviparous sponge Cliona viridis: coupling of larval abundance and adult distribution. Mar Biol 137:783–790
Mariani S, Piscitelli M, Uriz MJ (2001) Temporal and spatial co-occurrence in spawning and larval release of Cliona viridis (Porifera: Hadromerida). J Mar Biolog Assoc UK 81:365–367
Mariani S, Uriz MJ, Turon X (2003) Methodological bias in the estimations of important meroplanktonic components from near-shore bottoms. Mar Ecol Prog Ser 253:67–75
Mariani S, Uriz MJ, Turon X (2005a) The dynamics of sponge larvae assemblages from northwestern Mediterranean nearshore bottms. J Plankt Res 27:249–262
Mariani S, Alcoverro T, Uriz M-J, Turon X (2005b) Early life histories in the bryozoan Scizobrachiella sanguinea: a case study. Mar Biol 147:735–745
Metaxas A (2001) Behaviour in flow: perspectives on the distribution and dispersion of meroplanktonic larvae in the water column. Can J Fish Aquat Sci 58:86–98
Olson RR (1985) The consequences of short-distance larval dispersal in a sessile marine invertebrate. Ecology 66:30–39
Olson RR, McPherson R (1987) Potential vs. realized larval dispersal: fish predation on larvae of the ascidian Lissoclinum patella (Gottshaldt). J Exp Mar Biol Ecol 110:245–256
Paris CB, Cowen RK (2004) Direct evidence of a biophysical retention mechanism for coral reef fish larvae. Limnol Oceanogr 49:1964–1979
Raimondi PT (1991) Settlement behavior of Chtamalus anisopoma larvae largely determines the adult distribution. Oecologia 85:349–360
Stoner DS (1990) Recruitment of a tropical colonial ascidian: relative importance of pre-settlement vs. post settlement processes. Ecology 71:1682–1690
Stoner DS (1992) Vertical distribution of a colonial ascidian on a coral reef: the roles of larval dispersal and life-history variation. Am Nat 139:802–824
Svane I, Young CM (1989) The ecology and behaviour of ascidian larvae. Ocean Mar Biol Annu Rev 27:45–90
Underwood AJ, Keough (2002) Supply-side ecology. The nature and consequences of variations in recruitment of intertidal organisms. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer, Sunderland, pp 183–200
Uriz MJ, Rosell D, Martin D (1992) The sponge population of the Cabrera archipelago (Balearic Islands): characteristics, distribution, and abundance of the most representative species. PSZNI Mar Ecol 13:101–117
Uriz MJ, Turon X, Becerro MA, Galera J, Lozano J (1996) Feeding deterrence in sponges. The role of toxicity, physical defences, energetic contents, and life-history stage. J Exp Mar Biol Ecol 205:187–204
Uriz MJ, Maldonado M, Turon X, Martí R (1998) How do reproductive output, larval behaviour, and recruitment contribute to adult spatial patterns in Mediterranean encrusting sponges? Mar Ecol Prog Ser 167:137–148
Uriz MJ, Turon X, Becerrro MA (2001) Morphology and ultrastructure of the swimming larvae of Crambe crambe (Demospongiae, Poecillosclerida). Invertebr Biol 120:295–307
Young CM, Chia FS (1987) Abundance and distribution of pelagic larvae as influenced by predation, behavior, and hydrogeographic factors. In: Giese AC (ed) Reproduction of marine invertebrates, vol 9. Blackwell, Palo Alto, pp 385–464
Zea S (1993) Recruitment of Demosponges (Porifera, Demospongiae) in rocky and coral reef habitats of Santa Marta, Colombian Carribean. PSZNI Mar Ecol 14:1–21
Acknowledgments
The study benefited from European Community funds (Marie Curie Fellowship to S.M) and a CTM 2004-05265 grant from the Spanish Ministry of Science and Technology. We thank I. Abreu and G. Carreras for assistance from the boat, R. Arthur for corrections, and other people for critical comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Attrill
Rights and permissions
About this article
Cite this article
Mariani, S., Uriz, MJ., Turon, X. et al. Dispersal strategies in sponge larvae: integrating the life history of larvae and the hydrologic component. Oecologia 149, 174–184 (2006). https://doi.org/10.1007/s00442-006-0429-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0429-9