Abstract
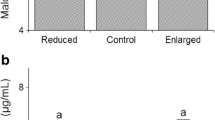
The allocation of resources to reproduction and survival is a central question of studies of life history evolution. Usually, increased allocation to current reproduction is paid in terms of reduced future reproduction and/or decreased survival. However, the proximal mechanisms underlying the cost of reproduction are poorly understood. Recently, it has been shown that increased susceptibility to oxidative stress might be one of such proximate links between reproduction and self-maintenance. Organisms possess a range of antioxidant defenses, including endogenously produced molecules (e.g., enzymes) and compounds ingested with food (e.g., carotenoids). If reproductive effort increases the production of reactive oxygen species, the availability of antioxidant defenses may partly or fully counteract the free-radical damages. One could, therefore, expect that the trade-off between reproduction and oxidative stress is modulated by the availability of antioxidant defenses. We tested this hypothesis in zebra finches. We manipulated reproductive effort by either allowing or preventing pairs to breed. Within each breeding or non-breeding group, the availability of antioxidant compounds was manipulated by supplementing or not supplementing the drinking water with carotenoids. We found that although birds in the breeding and non-breeding groups did not differ in their resistance to oxidative stress (the breakdown of red blood cells submitted to a controlled free-radical attack), one aspect of breeding effort (i.e., the number of eggs laid by birds in both breeding and non-breeding groups) was negatively correlated with resistance to oxidative stress only in birds that did not benefit from a carotenoid-supplemented diet. This result therefore suggests that carotenoid availability can modulate the trade-off between reproduction and resistance to oxidative stress.




Similar content being viewed by others
References
Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G (2004a) Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett 7:363–368
Alonso-Alvarez C, Bertrand S, Devevey G, Gaillard M, Prost J, Faivre B, Sorci G (2004b) An experimental test of dose-dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am Nat 164:651–659
Badyaev AV, Hill GE (2000) Evolution of sexual dichromatism: contribution of carotenoid- versus melanin-based coloration. Biol J Linn Soc 69:153–172
Barnes AI, Partridge L (2003) Costing reproduction. Anim Behav 66:199–204
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Birkhead TR, Pellatt J, Hunter FM (1988) Extra-pair copulation and sperm competition in the zebra finch. Nature 334:60–62
Blache D, Prost M (1992) Free radical attack: biological test for human resistance capability. In: Ponnamperuma C, Gehrke CW (eds) A lunar-based chemical analysis laboratory. Deepak A, Hampton, pp 82–98
Blount JD, Metcalfe NB, Birkhead TR, Surai PF (2003) Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300:125–127
Burley N, Coopersmith CB (1987) Beak color preferences of Zebra finches. Ethology 76:133–151
Burley NT, Price DK, Zann RA (1992) Beak color, reproduction and condition effects in wild and domesticated zebra finches. Auk 109:13–23
Chew BP, Park JS (2004) Carotenoid action on the immune response. J Nutr 134:257S–261S
Chidambara Murthy KN, Vanitha A, Rajesha J, Mahadeva Swamy M, Sowmya PR, Ravishankar GA (2005) In vivo antioxidant activity of carotenoids from Dunaliella salina—a green microalga. Life Sci 76:1381–1390
Collins SA, Ten Cate C (1996) Does beak colour affect female preference in zebra finches? Anim Behav 52:105–112
Cotton S, Fowler K, Pomiankowski A (2004) Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc R Soc Lond B 271:771–783
Eberhardt LS (1994) Oxygen consumption during singing by male Carolina wrens Thryothorus ludovicianus. Auk 111:124–130
El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, Young AJ (2004) Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys 430:37–48
Endler JA (1980) Natural selection on color patterns in Poecilia reticulata. Evolution 34:76–91
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Girard A, Madani S, El Boustani ES, Belleville J, Prost J (2005) Changes in lipid metabolism and antioxidant defense status in spontaneously hypertensive rats and Wistar rats fed a diet enriched with fructose and saturated fatty acids. Nutrition 21:240–248
Girodon F, Blache D, Monget AL, Lombart M, Brunet-Lecompte P, Arnaud J (1997) Effect of a two-year supplementation with low doses of antioxidant vitamins and/or minerals in elderly subjects on levels of nutrients and antioxidant defense parameters. J Am Coll Nutr 16:357–365
Griffith SC (2000) A trade-off between reproduction and a condition-dependent sexually selected ornament in the house sparrow Passer domesticus. Proc R Soc Lond B 267:1115–1119
Gustafsson L, Qvarnström A, Sheldon BC (1995) Trade-offs between life-history traits and a secondary sexual character in male collared flycatchers. Nature 375:311–313
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Hartley RC, Kennedy MW (2004) Are carotenoids a red herring in sexual display? Trends Ecol Evol 19:353–354
Hill G (1990) Female house finches prefer colourful males: sexual selection for a condition-dependent trait. Anim Behav 40:563–572
Hill G (1991) Plumage coloration is a sexually selected indicator of male quality. Nature 350:337–339
Kiokias S, Gordon MH (2004) Antioxidant properties of carotenoids in vitro and in vivo. Food Rev Int 20:99–121
Kodric-Brown A (1985) Female preference and sexual selection for male coloration in the guppy (Poecilia reticulata). Behav Ecol Sociobiol 17:199–206
Kodric-Brown A (1989) Dietary carotenoids and male mating success in the guppy: an environmental component to female choice. Am Nat 124:309–323
Lesgards JF, Durand P, Lassare M, Stocker P, Lesgard G, Lanteaume A (2002) Assessment of lifestyle effects on the overall antioxidant capacity of healthy subjects. Environ Health Perspect 110:479–487
Lozano GA (1994) Carotenoids, parasites, and sexual selection. Oikos 70:309–311
Martin OY, Hosken DJ (2004) Copulation reduces male but not female longevity in Saltella sphondylli (Diptera:Sepsidae). J Evol Biol 17:357–362
McGraw KJ, Ardia DR (2003) Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am Nat 162:704–712
Navara KJ, Hill GE (2003) Dietary carotenoid pigments and immune function in a songbird with extensive carotenoid-based plumage coloration. Behav Ecol 14:909–916
Partridge L, Andrews R (1985) The effect of reproductive activity on the longevity of male Drosophila melanogaster is not caused by an acceleration of ageing. J Insect Physiol 31:393–395
Pieri C, Moroni F, Marra M (1996) Food restriction increases the protection of erythrocytes against the hemolysis induced by peroxyl radicals. Mech Ageing Dev 87:15–23
Prior RL, Cao G (1999). In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med 27:1173–1181
Reznick D (1985) Costs of reproduction: an evaluation of the empirical evidence. Oikos 44:257–267
Reznick D (1992) Measuring the costs of reproduction. Trends Ecol Evol 7:42–45
Roff DA (1992) The evolution of life histories: theory and analysis. Chapman & Hall, New York
Rojas Wahl RU, Zeng L, Madison SA, De Pinto RL, Shay BJ (1998) Mechanistic studies on the decomposition of water soluble azo-radical-initiators. J Chem Soc Perkin Trans 2 9:2009–2018
Rose MR, Bradley TJ (1998) Evolutionary physiology of the cost of reproduction. Oikos 83:443–451
Salmon AB, Marx DB, Harshman LG (2001) A cost of reproduction in Drosophila melanogaster: stress susceptibility. Evolution 55:1600–1608
SAS Institute (2001) SAS/STAT Software: changes and enhancements, Version 8.2. SAS Publishing, North Carolina
Schabath MB, Grossman HB, Delclos GL, Hernandez LM, Day RS, Davis BR, Lerner SP, Spitz MR, Wu X (2004) Dietary carotenoids and genetic instability modify bladder cancer risk. J Nutr 134:3362–3369
von Schantz TV, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc Lond B 266:1–12
Sheldon B, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H (1994) Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev 74:121–133
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Stocker P, Lesgards J-F, Vidal N, Chalier F, Prost M (2003) ESR study of a biological assay on whole blood: antioxidant efficiency of various vitamins. Biochem Biophys Acta 1621:1–8
Tamimi RM, Hankinson SE, Campos H, Spiegelman D, Zhang S, Colditz GA, Willett WC, Hunter DJ (2005) Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am J Epidemiol 161:153–160
Tinbergen JM, Verhulst S (2000) A fixed energetic ceiling to parental effort in the great tit? J Anim Ecol 69:323
Vehrencamp SL, Bradbury JW, Gibson RM (1989) The energetic cost of display in male sage grouse. Anim Behav 38:885–896
Wang Y, Salmon AB, Harshman LG (2001) A cost of reproduction: oxidative stress susceptibility is associated with increased egg production in Drosophila melanogaster. Exp Gerontol 36:1349–1359
Wiersma P, Selman C, Speakman JR, Verhulst S (2004) Birds sacrifice oxidative protection for reproduction. Proc R Soc Lond B Suppl 271:360–363
Zera AJ, Harshman LG (2001) The physiology of life-history trade-offs in animals. Annu Rev Ecol Syst 32:95–126
Acknowledgements
We are very grateful to Kemin France for kindly providing the carotenoids (Oro GloTM) used in this study. We thank the staff of the Station Biologique de Foljuif (École Normale Supérieure) for helping us to maintain the birds. Financial support was provided by the Ministère de la Recherche (ACI Jeunes Chercheurs to GS) and the Université de Bourgogne (BQR to GD, MG, JP and BF). CA-A was funded by Ministerio de Educación, Cultura y Deporte (Spain). This experiment complies with the current laws of France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Carol Vleck
Rights and permissions
About this article
Cite this article
Bertrand, S., Alonso-Alvarez, C., Devevey, G. et al. Carotenoids modulate the trade-off between egg production and resistance to oxidative stress in zebra finches. Oecologia 147, 576–584 (2006). https://doi.org/10.1007/s00442-005-0317-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0317-8