Abstract
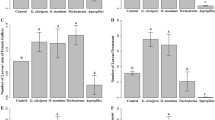
Feedback from community interactions involving mutualisms are a rarely explored mechanism for generating complex population dynamics. We examined the effects of two linked mutualisms on the population dynamics of a beetle that exhibits outbreak dynamics. One mutualism involves an obligate association between the bark beetle, Dendroctonus frontalis and two mycangial fungi. The second mutualism involves Tarsonemus mites that are phoretic on D. frontalis (“commensal”), and a blue-staining fungus, Ophiostoma minus. The presence of O. minus reduces beetle larval survival (“antagonistic”) by outcompeting beetle-mutualistic fungi within trees yet supports mite populations by acting as a nutritional mutualist. These linked interactions potentially create an interaction system with the form of an endogenous negative feedback loop. We address four hypotheses: (1) Direct negative feedback: Beetles directly increase the abundance of O. minus, which reduces per capita reproduction of beetles. (2) Indirect negative feedback: Beetles indirectly increase mite abundance, which increases O. minus, which decreases beetle reproduction. (3) The effect of O. minus on beetles depends on mites, but mite abundance is independent of beetle abundance. (4) The effect of O. minus on beetles is independent of beetle and mite abundance. High Tarsonemus and O. minus abundances were strongly correlated with the decline and eventual local extinction of beetle populations. Manipulation experiments revealed strong negative effects of O. minus on beetles, but falsified the hypothesis that horizontal transmission of O. minus generates negative feedback. Surveys of beetle populations revealed that reproductive rates of Tarsonemus, O. minus, and beetles covaried in a manner consistent with strong indirect interactions between organisms. Co-occurrence of mutualisms embedded within a community may have stabilizing effects if both mutualisms limit each other. However, delays and/or non-linearities in the interaction systems may result in large population fluctuations.





Similar content being viewed by others
References
Addicott JF (1986) On the population consequences of mutualism. In: Diamond J, Case T (eds) Community ecology. Harper and Row, New York, pp 425–436
Ayres MP, Wilkens RT, Ruel JJ, Lombardero MJ, Vallery E (2000) Nitrogen budgets of phloem feeding bark beetles with and without symbiotic fungi (Coleoptera: Scolytidae). Ecology 81:2198–2210
Barras SJ (1970) Antagonism between Dendroctonus frontalis and the fungus Ceratocystis minor. Annu Entomol Soc Am 63:1187–1190
Barras SJ (1972) Improved White’s solution for surface sterilization of pupae of Dendroctonus frontalis. J Econ Entomol 65:1504
Barras SJ (1973) Reduction of progeny and development in the southern pine beetle following removal of symbiotic fungi. Can Entomol 105:1295–1299
Barras SJ, Perry TJ (1975) Interrelationships among microorganisms, bark or ambrosia beetles, and woody plant tissue: an annotated bibliography, 1965–1974. USDA Forest Service, Southern Forest Experiment Station, Gen Tech Report SO−10
Berryman AA (1972) Resistance of conifers to invasion of bark beetle-fungus associations. BioScience 22:598–602
Berryman AA (2002) Populations: a central concept for ecology? Oikos 97:439–442
Bridges JR (1983) Mycangial fungi of Dendroctonus frontalis (Coleoptera: Scolytidae) and their relationship to beetle population trends. Environ Entomol 12:858–861
Bridges JR, Moser JC (1983) Role of two phoretic mites in transmission of bluestain fungus, Ceratocystis minor. Ecol Entomol 8:9–12
Clark EW, Richmond JA (1977) Variations of free and triglyceride fatty acids in phloem of Pinus taeda infected by Ceratocystis minor. Turrialba 27:377–383
Coulson RN (1980) Population dynamics. In: Thatcher RC, Searcy J, Coster J, Hertel G (eds) The southern pine beetle. USDA Tech Bull 1631, pp 71–105
Craighead FC (1925) Bark beetle epidemics and rainfall deficiency. J Econ Entomol 18:557–586
Crawly MJ (1990) The population dynamics of plants. Phil Trans R Soc Lond B Biol Sci 330:125–140
Cronin JT, Reeve JD, Wilkens R, Turchin P (2000) The pattern and range of movement of a checkered beetle predator relative to its bark beetle prey. Oikos 90:127–138
DeAngelis JD, Hodges JD, Nebeker TE (1986) Phenolic metabolies of Ceratocystis minor from laboratory cultures and their effects on transpiration in loblolly pine seedlings. Can J Bot 64:151–155
Dean AM (1983) A simple model of mutualism. Am Nat 121:409–417
Dickman CR (1992) Commensal and mutualistic interactions among terrestrial vertebrates. Trends Ecol Evol 7:194–197
Eckhardt LG, Jones JP, Klepzig KD (2004) Pathogenicity of Leptographium species associated with loblolly pine decline. Plant Dis 88:1174–1178
Ewald P (1995) The evolution of virulence: a unifying link between parasitology and ecology. J Parasitol 81:659–669
Fargo WS, Coulson RN, Gagne JA, Foltz JL (1979) Correlation of southern pine beetle attack density, oviposition, and generation survival with host tree characteristics and preceding beetle life stage within the host. Environ Entomol 8:624–628
Foltz JL, Mayyasi AM, Hain FP, Coulson RN, Martin WC (1976) Egg-gallery length relationship and within-tree analysis for southern pine beetle, Dendroctonus frontalis. Zimm (Coleoptera: Scolytidae). Can Entomol 108:341–352
Goldhammer DS, Stephen FM, Paine TD (1989) Average radial growth rate and chlamydospore production of Ceratocystis minor, Ceratocystis minor var. barrasii, and SJB 122 in culture. Can J Bot 67:3498–3505
Goldhammer DS, Stephen FM, Paine TD (1990) The effect of the fungi Ceratocystis minor Hunt, Ceratocystis minor. Hunt var Barrasii Taylor, and SJB 122 on reproduction of the southern pine beetle, Dendroctonus frontalis Zimmerman (Coleoptera: Scolytidae). Can Entomol 122:407–418
Hain FP (1980) Sampling and predicting population trends. In: Thatcher RC, Searcy J, Coster J, Hertel G (eds) The southern pine beetle. USDA Tech Bull 1631, pp 107–135
Happ GM, Happ CM, Barras SJ (1976) Bark beetle-fungal symbiosis. II. Fine structure of a basidiomycetous ecosymbiont of the southern pine beetle. Can J Bot 54:1049–1062
Hemingway RW, McGraw GW, Barras SJ (1977) Polyphenols in Ceratocystis minor infected Pinus taeda: Fungal metabolites, phloem and xylem phenols. Agric Food Chem 25:717–722
Herre EA, Knowlton N, Mueller UG, Rehner SA (1999) The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol Evol 14:49–53
Hofstetter RW, Klepzig KD, Moser JC, Ayres MP (2005a) Seasonal dynamics of mites and fungi and their interaction with southern pine beetle. Env Ent (in press)
Hofstetter RW, Mahfous J, Klepzig KD, Ayres MP (2005b) Effects of tree phytochemistry on the interactions between endophloedic fungi associated with the southern pine beetle. J Chem Ecol 31:551–572
Holbrook SJ, Schmitt RJ (2004) Population dynamics of a damselfish: effects of a competitor that also is an indirect mutualist. Ecology 85:979–985
Holland JN, DeAngelis DL (2001) Population dynamics and the stability of obligate pollination mutualisms. Oecologia 126:575–586
Hsiau PTW (1996) The taxonomy and phylogeny of the mycangial fungi from Dendroctonus brevicomis and Dendroctonus frontalis (Coleoptera: Scolytidae). Dissertation, Iowa State University, Ames, Iowa
Jacobs K, Wingfield MJ (2001) Leptographium Species: tree pathogens, insect associates and agents of blue-stain. American Phytopathological Society Press, St Paul
Jacobs K, Kirisits T (2003) Ophiostoma kryptum sp. nov. from Larix decidua and Picea abies in Europe, similar to O. minus. Mycol Res 107:1231–1242
Jones CG, Ostfeld RW, Richard MP, Schauber EM, Wolff JO (1998) Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science 279:1023–1026
Kalkstein LS (1976) Effects of climatic stress on outbreaks of the southern pine beetle. Environ Entomol 5:653–658
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualisms: the conservation of plant-pollinator interactions. Annu Rev Ecol Syst 29:83–112
Klepzig KD (1998) Competition between a biological control fungus, Ophiostoma piliferum, and symbionts of the southern pine beetle. Mycologia 90:69–75
Klepzig KD, Wilkens RT (1997) Competitive interactions among symbiotic fungi of the southern pine beetle. Appl Environ Microbiol 63:621–627
Klepzig KD, Moser JC, Lombardero MJ, Hofstetter RW, Ayres MP (2001) Symbiosis and competition: complex interactions among beetles, fungi, and mites. Symbiosis 30:83–96
Klepzig KD, Flores-Otero J, Hofstetter RW, Ayres MP (2004) Effects of available water on growth and competition of southern pine beetle associated fungi. Mycol Res 108:183–188
Klepzig KD, Six DL (2004) Bark beetle-fungal symbiosis: context dependency in complex associations. Symbiosis 37:189–205
Kopper BJ, Klepzig KD, Raffa KF (2004) Components of antagonism and mutualism in Ips pini-fungal interactions: relationship to a life history of colonizing highly stressed and dead trees. Environ Entomol 33:28–34
Krokene P, Solheim H (1998) Pathogenicity of four blue-stain fungi associated with aggressive and nonaggressive bark beetles. Phytopathology 88:39–44
Leach JG, Orr LW, Christensen C (1934) The interrelationships of bark beetles and blue-staining fungi in felled Norway pine timber. J Agric Res 49:315–342
Levins R, Schultz BB (1996) Effects of density dependence, feedback and environmental sensitivity on correlations among predators, prey and plant resources: models and practical implications. J Anim Ecol 65:802–812
Lima M, Jaksic FM (1999) Population dynamics of three Neotropical small mammals: time series models and the role of delayed density-dependence in population irruptions. Aust J Ecol 24:25–34
Lombardero MJ, Klepzig KD, Moser JC, Ayres MP (2000) Biology, demography and community interactions of Tarsonemus (Acrina: Tarsonemidae) mites phoretic on Dendroctonus frontalis (Coleoptera: Scolytidae). Agric For Entomol 2:193–202
Lombardero MJ, Ayres MP, Hofstetter RW, Moser JC, Klepzig KD (2003) Strong indirect interactions of Tarsonemus mites (Acrina: Tarsonemidae) and Dendroctonus frontalis (Coleoptera: Scolytidae). Oikos 102:243–252
May RM (1976a) Simple mathematical models with very complicated dynamics. Nature 261:459–467
May RM (1976b) Theoretical ecology: principles and applications. Blackwell, Oxford
May RM (1982) Mutualistic interactions among species. Nature 296:803–804
Menge BA (2000) Testing the relative importance of positive and negative effects on community structure. Trends Ecol Evol 15:46–47
Moser JC (1985) Use of sporothecia by phoretic Tarsonemus mites to transport ascospores of coniferous bluestain fungi. Trans Br Mycol Soc 84:750–753
Moser JC, Bridges JR (1986) Tarsonemus (Acarina: Tarsonemidae) mites phoretic on the southern pine beetle (Coleoptera: Scolytidae): attachment sites and numbers of bluestain (Ascomycetes: Ophiostomataceae) ascospores carried. Proc Entomol Soc Wash 88:297–299
Moser JC, Perry TJ, Bridges JR, Yin H-F (1995) Ascospore dispersal of Ceratocystiopsis ranaculosus, a mycangial fungus of the southern pine beetle. Mycologia 87:84–86
Moser JC, Macias-Samano JE (2000) Tarsonemid mite associates of Dendroctonus frontalis (Coleoptera: Scolytidae): implications for the historical biogeography of D. frontalis. Can Entomol 132:765–771
Owen DR, Lindahl KQ, Wood DL (1987) Pathogenicity of fungi isolated from Dendroctonus valens, D. brevicomis and D. ponderosae to ponderosa pine seedlings. Phytopathology 77:631–636
Perry TJ (1991) A synopsis of the taxonomic revisions in the genus Ceratocystis including a review of blue-staining species associated with Dendroctonus bark beetles. Gen Tech Report SO-86, New Orleans, LA
Price TS, Dogget HC, Pye JM, Smith B (1997) A history of southern pine beetle outbreaks in the southeastern US. Georgia Forestry Commission, Macon
Reeve JR (1997) Predation and bark beetle dynamics. Oecologia 112:48–54
Reeve JR, Turchin P (2002) Evidence for predator-prey cycles in a bark beetle. In: Berryman AA (ed) Population cycles the case for trophic interactions. Oxford University Press, New York, pp 92–108
Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmanek M (2000) Plant invasions—the role of mutualisms. Biol Rev 75:65–93
Ringel MS, Hu HH, Anderson G (1996) The stability and persistence of mutualisms embedded in community interactions. Theor Popul Biol 50:281–297
Seifert KA (1993) Sapstain of commercial lumber by species of ophiostoma and ceratocystis. In: Wingfield MJ, Seifert KA, Webber JF (eds) Ceratocystis and ophiostoma: taxonomy, ecology and pathogenicity. American Phytopathology Press, St. Paul, pp 141–151
Six DL, Paine TD (1998) Effects of mycangial fungi and host tree species on progeny survival and emergence of Dendroctonus ponderosae (Coleoptera: Scolytidae). Environ Entomol 27:1393–1401
Solheim H (1986) Species of Ophiostomataceae isolated from Picea abies infested by the bark beetle Ips typographus. Nord J Bot 6:199–207
Stanton ML (2003) Interacting guilds: moving beyond the pairwise perspective of mutualisms. Am Nat 162:S10–S23
Strobel GA, Lanier GN (1981) Dutch elm disease. Sci Am 245:40–50
Thatcher RC, Searcy JL, Coster JE, Hertel GD (1980) The southern pine beetle. Technical Bulletin 1631, United States Department of Agriculture Forest Service, Combined Forest Pest Reserch and Development Program, Pineville, LA. 267 pp
Turchin P, Lorio PL, Taylor AD, Billings RF (1991) Why do populations of southern pine beetles (Coleoptera: Scolytidae) fluctuate? Environ Entomol 20:401–409
Turchin P, Taylor AD (1992) Complex dynamics in ecological time series. Ecology 73:289–305
Turchin P, Taylor AD, Reeve JD (1999) Dynamical role of predators in population cycles of a forest insect: an experimental test. Science 285:1068–1071
Ungerer MJ, Ayres MP, Lombardero MJ (1999) Climate and the northern distribution limits of Dendroctonus frontalis Zimmermann (Coleoptera: Scolytidae). J Biogeog 26:1133–1145
Waser NW, Price MV, Brody AK, Campbell DR (2000) Pollination success and plant population size: how strong are the links? Bull Ecol Soc Am 85:S227
Wilson DS (1986) Adaptive indirect effects. In: Diamond J, Case T (eds) Community ecology. Harper and Row, New York, pp 437–444
Wootton JT (1993) Indirect effects and habitat use in an intertidal community: interaction chains and interaction modifications. Am Nat 141:71–89
Wullschlager SD, McLaughlin SB, Ayres MP (2004) High-resolution analysis of stem increment and sap flow for loblolly pine trees attacked by southern pine beetle. Can J For Res 34: (in press)
Yearian WC, Gouger RJ, Wilkinson RC (1972) Effects of the bluestain fungus, Ceratocystis ips, on development of Ips bark beetles in pine bolts. Annu Entomol Soc Am 65:481–48
Acknowledgements
We thank Matt Babineau, Dan Braden, Julia Brant, Debbie Cronin, Johnny Fryar, Dan Hogan, Karen London, Sharon Martinson, Jason Moan, Chris Steiner, Erich Vallery, Jessica Veysey and Tiina Ylioja for their assistance in the field and laboratory. Thanks to the researchers and staff at the US Forest Service in Pineville LA, and Bankhead, Homochitto and Oakmulgee Ranger Districts. The manuscript benefited from comments by Anurag Agrawal, Bill Mattson, Mark McPeek, David Peart, and Tiina Yliola. Research was supported by the US Forest Service, NRI CGPs #9835302, #2001-35302-09921, Dartmouth College Graduate Fellowship to R.W. Hofstetter, and National Science Foundation grant DEB 950923 to P. Turchin and J. D. Reeve.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jay Rosenheim
Electronic supplementary material
Appendix B
Appendix B
Table 4
Rights and permissions
About this article
Cite this article
Hofstetter, R.W., Cronin, J.T., Klepzig, K.D. et al. Antagonisms, mutualisms and commensalisms affect outbreak dynamics of the southern pine beetle. Oecologia 147, 679–691 (2006). https://doi.org/10.1007/s00442-005-0312-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0312-0