Abstract
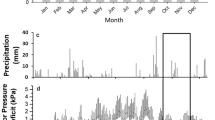
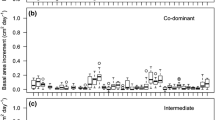
The carbon isotope signature (δ13C) of foliar cellulose from sunlit tops of trees typically becomes enriched as trees of the same species in similar environments grow taller, indicative of size-related changes in leaf gas exchange. However, direct measurements of gas exchange in common environmental conditions do not always reveal size-related differences, even when there is a distinct size-related trend in δ13C of the very foliage used for the gas exchange measurements. Since δ13C of foliage predominately reflects gas exchange during spring when carbon is incorporated into leaf cellulose, this implies that gas exchange differences in different-sized trees are most likely to occur in favorable environmental conditions during spring. If gas exchange differs with tree size during wet but not dry conditions, then this further implies that environmental sensitivity of leaf gas exchange varies as a function of tree size. These implications are consistent with theoretical relationships among height, hydraulic conductance and gas exchange. We investigated the environmental sensitivity of gas exchange in different-sized Douglas-fir (Pseudotsuga menziesii) via a detailed process model that specifically incorporates size-related hydraulic conductance [soil–plant–atmosphere (SPA)], and empirical measurements from both wet and dry periods. SPA predicted, and the empirical measurements verified, that differences in gas exchange associated with tree size are greatest in wet and mild environmental conditions and minimal during drought. The results support the hypothesis that annual net carbon assimilation and transpiration of trees are limited by hydraulic capacity as tree size increases, even though at particular points in time there may be no difference in gas exchange between different-sized trees. Maximum net ecosystem exchange occurs in spring in Pacific Northwest forests; therefore, the presence of hydraulic limitations during this period may play a large role in carbon uptake differences with stand-age. The results also imply that the impacts of climate change on the growth and physiology of forest trees will vary depending on the age and size of the forest.









Similar content being viewed by others
References
Barnard H, Ryan MG (2003) A test of the hydraulic limitation hypothesis in fast-growing Eucalyptus saligna. Plant Cell Environ 26:1235–1245
Becker P, Meinzer FC, Wullschleger SD (2000) Hydraulic limitation of tree height: a critique. Funct Ecol 14(1):4–11
Bond BJ, Ryan MG (2000) Comment on ’Hydraulic limitation of tree height: a critique’ by Becker, Meinzer and Wullschleger. Funct Ecol 14(1):37–40
Brugnoli EA, Scartazza M, Lauteri MC, Monteverdi MC, Maguas C (1998) Carbon isotope discrimination in structural and non-structural carbohydrates in relation to productivity and adaptation to unfavourable conditions. In: Griffiths G (ed) Stable Isotopes. BIOS Scientific Publishers Ltd., Oxford, pp 133–146
Dawson TE (1996) Determining water use by trees and forests from isotopic, energy balance and transpiration analyses: the role of tree size and hydraulic lift. Tree Physiol 16:263–272
Domec J-C, Warren J, Meinzer F, Brooks J, Coulombe R (2004) Native root xylem embolism and stomatal closure in stands of Douglas-fir and ponderosa pine: mitigation by hydraulic redistribution. Oecologia 141:7–16
Ehleringer JR (1993) Carbon and water relations in desert plants: an isotopic perspective. In: Ehleringer JR, Hall AE, Farquhar GD (eds) Stable isotopes and plant carbon-water relations. Academic, San Diego, pp 155–172
Ehleringer JR, Hall AE, Farquhar GD (1993) Stable isotopes and plant carbon-water relations. Academic, San Diego
Ellsworth DS (2000) Seasonal CO2 assimilation and stomatal limitations in a Pinus taeda canopy. Tree Physiol 20:435–445
Escalona JM, Flexas J, Medrano H (1999) Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Aust J Plant Physiol 26:421–433
Evans JR, von Caemmerer S (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110:339–346
Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13:281–292
Ewers BE, Oren R, Sperry JS (2000) Influence of nutrient versus water supply on hydraulic architecture and water balance in Pinus taeda. Plant Cell Environ 23:1055–1066
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Franklin JF, DeBell DS (1988) Thirty-six years of tree population change in an old-growth Pseudotsuga-Tsuga forest. Can J For Res 18:633–639
Gillon JS, Yakir D (2000) Internal conductance to CO2 diffusion and C18 OO discrimination in C3 leaves. Plant Physiol 123:201–213
Gower ST, McMurtrie RE, Murty D (1996) Aboveground net primary production decline with stand age: potential causes. Trends in Ecology and Evolution 11(9):378–382
Hacke UG, Sperry JS, Ewers B, Schäfer KVR, Oren R (2000) Influence of soil porosity on water use in Pinus taeda. Oecologia. 124(4):495–505
Hanba YT, Miyazawa S-I, Terashima I (1999) The influence of leaf thickness on CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm temperate forests. Funct Ecol 13:632–639
Hubbard RM, Bond BJ, Ryan MG (1999) Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiol 19:165–172
Hubbard RM, Ryan MG, Stiller V, Sperry JS (2001) Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ 24:113–121
Kaufmann MR (1982) Leaf conductance as a function of photosynthetic photon flux density and absolute humidity difference from leaf to air. Plant Physiol 69:1018–1022
Koch G, Sillett S, Jennings G, Davis S (2004) The limits to tree height. Nature 428:851–854
Kolb TE, Stone JE (2000) Differences in leaf gas exchange and water relations among species and tree sizes in an Arizona pine-oak forest. Tree Physiol 20:1–12
Law BE, Williams M, Anthoni PM, Baldocchi DD, Unsworth MH (2000) Measuring and modelling seasonal variation of carbon dioxide and water vapour exchange of a pinus ponderosa forest subject to soil water deficit. Global Change Biol 6:1–18
Lewis JD, McKane RB, Tingey DT, Beedlow PA (2000) Vertical gradients in photosynthetic light response within an old-growth Douglas-fir and western hemlock canopy. Tree Physiol 20:447–456
Licata J (2003) Structural and physiological changes with stand age: use of a process-based model to compare carbon and water fluxes in young and old-growth Douglas-fir/Western Hemlock forest stands. MSc Thesis, Oregon State University
Lloyd J, Syvertsen JP, Kriedemann P, Farquhar GD (1992) Low conductances for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant Cell Environ 15:873–889
Magnani F, Mencuccini M, Grace J (2000). Age-related decline in stand productivity: the role of structural acclimation under hydraulic constraints. Plant Cell Environ 23(3):251–264
Martin B, Ruiz-Torres NA (1992) Effects of water-deficit stress on photosynthesis, its components and component limitations, and on water use efficiency in wheat (Triticum aestivum L.). Plant Physiol 100:733–739
McDowell NG, Phillips N, Lunch CK, Bond BJ, Ryan MG (2002a) An investigation of hydraulic limitation and compensation in large, old Douglas-fir trees. Tree Physiol 22:763–774
McDowell NG, Barnard H, Bond BJ, Hinckley T, Hubbard R, Ishii H, Köstner B, Meinzer FC, Marshall JD, Magnani F, Phillips N, Ryan MG, Whitehead D (2002b) The relationship between tree height and leaf area:sapwood area ratio. Oecologia 132:12–20
McDowell NG, Bowling DR, Schauer A, Bond BJ, Irvine J, Law BE, Ehleringer JR (2004) Associations between the carbon isotope content of ecosystem respiration, water availability, and canopy conductance. Global Change Biol 10:1767–1784
McNaughton KG, Jarvis PG (1991) Effects of spatial scale on stomatal control of transpiration. Agric For Meteorol 54:270–301
Meinzer FC, Goldstein G, Grantz DA(1993) Carbon isotope discrimination and gas exchange in coffee during adjustment to different soil moisture regimes. In: Ehleringer JR, Hall AE, Farquhar GD (eds) Stable isotopes and plant carbon-water relations. Academic, San Diego, pp 327–348
Meinzer FC, Clearwater MJ, Goldstein G (2001) Water transport in trees: current perspectives, new insights, and some controversies. Environ Exp Bot 45:239–262
Mencuccini M (2003) The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ 26:163–182
Mencuccini M, Grace J (1996a) Developmental patterns of above-ground hydraulic conductance in a Scots pine (Pinus sylvestris L.) age sequence. Plant Cell Environ 19:939–948
Mencuccini M, Grace J (1996b) Hydraulic conductance, light interception and needle nutrient concentration in Scots pine stands and their relations with net primary productivity. Tree Physiol 16:459–468
Mencuccini M, Magnani F (2000) Comment on ’Hydraulic limitation of tree height: a critique’ by Becker, Meinzer and Wullschleger. Funct. Ecol 14(1):135–136
Midgley JJ (2003) Is bigger better in plants? The hydraulic costs of increasing size in trees. Trends Ecol Evol 18:5–6
Murty D, McMurtrie R, Ryan MG (1996) Declining forest productivity in ageing forest stands: a modeling analysis of alternative hypotheses. Tree Physiol 16:187–200
Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schäfer K VR (1999) Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ 22:1515–1526
Panek JA, Waring RH (1997) Stable carbon isotopes as indicators of limitations to forest growth imposed by climate stress. Ecol Appl 7:854–863
Parker G, Harmon M, Lefsky M, Chen J, Van Pelt R, Weis S, Thomas S, Winner W, Shaw D, Franklin J (2004) Three-dimensional structure of an old-growth Pseudotsuga-tsuga canopy and its implications for radiation balance, microclimate, and gas exchange. Ecosystems 7:440–453
Paw U KT, Falk M, Suchanek TH, Ustin SL, Chen J, Park Y-S, Winner WE, Thomas SC, Hsiao TC, Shaw RH (2004) Carbon dioxide exchange between an old-growth forest and the atmosphere. Ecosystems 7:513–524
Phillips N, Bond BJ, McDowell NG, Ryan MG (2002) Canopy and hydraulic conductance in young, mature and old Douglas-fir trees. Tree Physiol 22:205–211
Phillips N, Ryan MG, Bond BJ, McDowell N, Hinckley T, Čermák J (2003) Reliance on stored water increases with tree size in three species in the Pacific Northwest. Tree Physiol 23:237–245
Pothier D, Margolis HA, Waring RH (1989) Patterns of change of saturated sapwood permeability and sapwood conductance with stand development. Can J For Res 19:432–439
Ryan MG, Yoder BJ (1997) Hydraulic limits to tree height and tree growth. Bioscience. 47(4):235–242
Ryan MG, Binckley D, Fownes JH (1997) Age-related decline in forest productivity: patterns and process. Adv Ecol Res 27:213–262
Ryan MG, Bond BJ, Law BE, Hubbard RM, Woodruff D, Cienciala E, Kucera J (2000) Transpiration and whole-tree conductance in ponderosa pine trees of different heights. Oecologia. 124:553–560
Ryan MG, Binkley D, Fownes J, Giardina C, Senock R (2004) An experimental test of the causes of forest growth decline with stand age. Ecol Monogr 74:393–414
Schäfer KVR, Oren R, Tenhunen JD (2000) The effect of tree height on crown level stomatal conductance. Plant Cell Environ 23:365–375
Schoettle A (1994) Influence of tree size on shoot structure and physiology of Pinus contorta and Pinus aristata. Tree Physiol 14:1055–1068
Sperry JS, Alder NN, Eastlack SE (1993) The effect of reduced hydraulic conductance on stomatal conductance and xylem cavitation. J Exp Bot 44:1075–1082
Sperry JS, Adler FR, Campbell GS, Comstock JP (1998) Limitation of plant water use by rhizosphere and xylem conductance: results from a model. Plant Cell Environ 21:347–359
Sperry JS, Hacke U, Oren R, Comstock J (2002) Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ 25:251–263
Thomas S, Winner WE (2000) Leaf area index of an old-growth Douglas-fir forest estimated from direct structural measurements in the canopy. Can J For Res 30:1922–1930
Waring RH, Running SW (1978) Sapwood water storage: its contribution to transpiration and effect upon water conductance through the stems of old-growth Douglas-fir. Plant Cell Environ 1:131–140
Whitehead D (1998) Regulation of stomatal conductance and transpiration in forest canopies. Tree Physiol 18:633–644
Whitehead D, Hinckley TM (1991) Models of water flux through forest stands: critical leaf and stand parameters. Tree Physiol 9:35–57
Whitehead D, Edwards WRN, Jarvis PG (1984) Conducting sapwood area, foliage area, and permeability in mature trees of Picea sitchensis and Pinus contorta. Can J For Res 14:940–947
Williams M, Rastetter EB, Fernandes DN, Goulden ML, Wofsy SC, Shaver GR, Melillo JM, Munger JW, Fan S-M, Nadelhoffer KJ (1996) Modelling the soil–plant–atmosphere continuum in a Quercus-Acer stand at Harvard Forest: the regulation of stomatal conductance by light, nitrogen and soil/plant hydraulic properties. Plant Cell Environ 19:911–927
Williams M, Bond BJ, Ryan MG (2001a) Evaluating different soil and plant hydraulic constraints on tree function using a model and water flux data from ponderosa pine. Plant Cell Environ 24:679–690
Williams M, Law BE, Anthoni PM, Unsworth M (2001b) Using a simulation model and ecosystem flux data to examine carbon-water interactions in ponderosa pine. Tree Physiol 21:287–298
Winner WE, Thomas S, Berry J, Bond BJ, Cooper CE, Hinckley TM, Ehleringer JR, Fessenden JE, Lamb B, McCarthy S, McDowell N, Phillips N, Williams M (2004) Canopy carbon gain and water use: Analysis of old-growth conifers in the Pacific Northwest. Ecosystems 7:482–497
Yoder BJ, Ryan MG, Waring RH, Schoettle AW, Kaufmann MR (1994) Evidence of reduced photosynthetic rates in old trees. For Sci 40(3):513–527
Yong, JWH, SC Wong , SC Farquhar GD (1997) Stomatal response to changes in vapour pressure difference between leaf and air. Plant Cell Environ 20:1213–1216
Acknowledgements
We appreciate the field assistance of Kate George, Tom Pypker, and Andy Schauer, and the help from the WRCCRF scientists and staff: Rick Meinzer, Ken Bible, Dave Shaw, Mark Creighton, Dave Braun, and Annette Hamilton. We also appreciate the two high quality reviews provided by anonymous reviewers. This research was funded in part by grants to Barbara J. Bond, Michael G. Ryan, and Mathew Williams from the Western Regional Center (WESTGEC) of the National Institute for Global Environmental Change (NIGEC) through the US Department of Energy (Cooperative Agreement# DE-FC03-90ER61010), and through Los Alamos National Laboratory’s “Laboratory Directed Research and Development” program. Any opinions, findings and conclusions or recommendations expressed herein are those of the authors and do not necessarily reflect the view of the DOE or LANL. All experiments complied with the laws of the USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Lawrence Flanagan
Rights and permissions
About this article
Cite this article
McDowell, N.G., Licata, J. & Bond, B.J. Environmental sensitivity of gas exchange in different-sized trees. Oecologia 145, 9–20 (2005). https://doi.org/10.1007/s00442-005-0104-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0104-6