Abstract
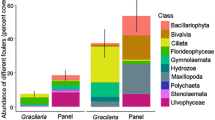
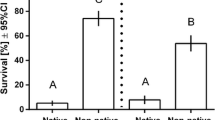
In this study, we examined genetic variation in resistance and tolerance to fouling organisms in the brown alga Fucus vesiculosus. We first grew 30 algal genotypes in the field, where we allowed fouling organisms to colonise the genotypes at natural levels. We then conducted a manipulative experiment, where we grew 20 genotypes of algae in aquaria with or without fouling organisms. We measured host resistance as the load of fouling organisms and tolerance as the slope of the regression of algal performance on fouling level. Fouling organisms decreased host growth and contents of phlorotannins and thus have the potential to act as selective agents on algal defenses. We found significant among-genotype variation in both resistance and tolerance to fouling. We did not find a trade-off between resistance and tolerance. We found a marginally significant cost of resistance, but no cost of tolerance. Our results thus indicate that both the tolerance and resistance of F. vesiculosus can evolve as a response to fouling and that the costs of resistance may maintain genetic variation in resistance.




Similar content being viewed by others
References
Agrawal AA, Strauss SY, Stout MJ (1999) Costs of induced responses and tolerance to herbivory in male and female fitness components of wild radish. Evolution 53:1093–1104
Bernstein GG, Jung N (1979) Selective pressure and coevolution in a kelp community in southern California. Ecol Monogr 49:335–355
Duffy JE, Harvilicz AM (2001) Species-specific impacts of grazing amphipods in an eelgrass-bed community. Mar Ecol Prog Ser 223:201–211
Fornoni J, Núñez-Farfán J (2000) Evolutionary ecology of Datura stramonium:genetic variation and costs for tolerance to defoliation. Evolution 54:789–797
Hillebrand H, Kahlert M (2001) Effect of grazing and nutrient supply on periphyton biomass and nutrient stoichiometry in habitat of different productivity. Limnol Oceanogr 46:1881–1898
Hillebrand H, Worm B, Lotze HK (2000) Marine microbenthic community structure regulated by nitrogen loading and grazing pressure. Mar Ecol Prog Ser 204:27–38
Honkanen T, Jormalainen V (2002) Within-alga integration and compensation: effects of simulated herbivory on growth and reproduction of the brown alga, Fucus vesiculosus. Int J Plant Sci 163:815–823
Hurd CL, Galvin RS, Norton TA, Dring MJ (1993) Production of hyaline hairs by intertidal species of Fucus (Fucales) and their role in phosphate uptake. J Phycol 29:160–165
Jennings JK, Steinberg PD (1997) Phlorotannins versus other factors affecting epiphyte abundance on the kelp Ecklonia radiata. Oecologia 109:461–473
Jernakoff P, Nielsen J (1997) The relative importance of amphipod and gastropod grazers in Posidonia sinuaosa meadows. Aquat Bot 56:183–202
Jormalainen V, Honkanen T, Koivikko R, Eränen J (2003) Induction of phlorotannin production in a brown alga: defense or resource dynamics? Oikos 103:640–650
Juenger T, Bergelson J (2000) The evolution of compensation to herbivory in scarlet gilia, Ipomopsis aggregata: herbivore-imposed natural selection and the quantitative genetics of tolerance. Evolution 54:764–777
Juenger T, Lennartsson T, Tuomi J (2000) The evolution of tolerane to damage in Gentianella campestris: natural selection and the quantitative genetics of tolerance. Evol Ecol 14:393–419
Karban R, Baldwin IT (1997) Induced responses to Herbivory. University of Chicago Press
Karez R, Engelbert S, Sommer U (2000) ‘Co-consumption’ and ‘protective coating’: two new proposed effects of epiphytes on their macroalgal hosts in mesograzer-epiphyte-host interactions. Mar Ecol Prog Ser 205:85–93
Keats DW, Wilton P, Maneveldt G (1994) Ecological significance of deep-layer sloughing in the eulittoral zone coralline alga, Spongites yendoi (Foslie) Chamberlain (Corallinaceae, Rhodophyta) in South Africa. J Exp Mar Biol Ecol 175:145–154
Kiirikki M (1996) Experimental evidence that Fucus vesiculosus (Phaeophyta) controls filamentous algae by the means of the whiplash effect. Eur J Phycol 31:61–66
Knight M, Parke M (1950) A biological study of Fucus vesiculosus L. and Fucus serratus L. J Mar Biol Ass UK 24:439–515
Koivikko R, Loponen J, Honkanen T, Jormalainen V (2005) Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J Chem Ecol 31:195–212
Lehvo A, Back S, Kiirikki M (2001) Growth of Fucus vesiculosus L. (Phaeophyta) in the northern Baltic proper: energy and nitrogen storage in seasonal environment. Botanica Marina 44:345–350
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models, 3rd edn. SAS Institute Inc., Cary
Littler MM, Littler DS (1999) Blade abondonment/proliferation: a novel mechanism for rapid epiphyte control in marine macrophytes. Ecology 80:1736–1746
Mauricio R (1998) Costs of resistance to natural enemies in field populations of the annual plant Arabidopsis thaliana. Am Nat 151:20–28
Mauricio R, Rausher MD, Burdick DS (1997) Variation in the defense strategies of plants: are resistance and tolerance mutually exclusive. Ecology 78:1301–1311
Neckles HA, Wetzel RL, Orth RJ (1993) Relative effects of nutrient enrichment and grazing on epiphyte-macrophyte (Zostera marina L.) dynamics. Oecologia 93:285–295
Paige KN, Whitham TG (1987) Overcompensation in response to mammalian herbivory: the advantage of being eaten. Am Nat 129:407–416
Pavia H, Cervin G, Lindgren A, Åberg P (1997) Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 157:139–146
Peckol P, Krane JM, Yates JL (1996) Interactive effects of inducible defense and resource availability on phlorotannins in the North Atlantic brown alga Fucus vesiculosus. Mar Ecol Prog Ser 138:209–217
Schmitt TM, Hay ME, Lindquist N (1995) Constraints on chemically mediated coevolution: multiple functions for seaweed secondary metabolites. Ecology 76:107–123
Schmitt TM, Lindquist N, Hay ME (1998) Seaweed secondary metabolites as antifoulants: effects of Dictyota spp. diterpenes on survivorship, settlement, and development of marine invertebrate larvae. Chemoecology 8:125–131
Schwaegerle KE, McIntyre H, Swingley C (2000) Quantitative genetics and the persistence of environmental effects in clonally propagated organisms. Evolution 54:452–461
Segerstråhle SG (1927) Quantitative studien über den tierbestand de Fucus-vegetation in den schären von Pellinge (an der südküste Finnlands). Societas Scientiarum Fennica Commentationes Biologicae III 2:1–14
Siemens DH, Lischke H, Maggiulli N Schürch S, Roy BA (2003) Cost of resistance and tolerance under competition: the defense-stress benefit hypothesis. Evol Ecol 17:247–263
Simms EL, Triplett J (1994) Costs and benefits of plant responses to disease: resistance and tolerance. Evolution 48:1973–1985
Steinberg PD, de Nys R. (2002) Chemical mediation of colonization of seaweed surfaces. J Phycol 38:621–629
Steinberg PD, de Nys R, Kjelleberg S (2002) Chemical cues for surface colonization. J Chem Ecol 28:1935–1951
Stinchcombe JR (2002) Environmental dependency in the expression of costs of tolerance to deer herbivory. Evolution 56:1063–1067
Stowe KA, Marquis RJ, Hochwender CG, Simms EL (2000) The evolutionary ecology of tolerance to consumer damage. Annu Rev Ecol Syst 31:565–595
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Strauss SY, Watson W, Allen MT (2003) Predictors of male and female tolerance to insect herbivory in Raphanus raphanistrum. Ecology 84:2074–2082
Tiffin P, Rausher MD (1999) Genetic constraints and selection acting on tolerance to herbivory in the common morning glory Ipomoea purpurea. Am Nat 154:700–716
Van Alstyne KL (1995) The comparison of three methods for quantifying brown algal polyphenolic compounds. J Chem Ecol 21:45–58
Van den Hoek C, Mann DG, Jahns HM (1998) Algae: an introduction to phycology. University Press, Cambridge
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. Blackwell Scientific Publications
Wikström SA, Pavia H (2003) Chemical settlement inhibition versus post-settlement mortality as an explanation for differential fouling of two congeneric seaweeds. Oecologia 138:223–230
Williams SL, Ruckelshaus MH (1993) Effects of nitrogen availability and herbivory on eelgrass (Zostera marina) and epiphytes. Ecology 74:904–918
Williams GA, Seed R (1992) Interactions between macrofaunal epiphytes and their host algae. In: John DM, Hawkins SJ, Price JH (eds) Plant–animal interactions in the Marine Benthos. Systematics Association Special Volume 46. Clarendon Press, GB-Oxford, pp 189–211
Worm B, Sommer U (2000) Rapid direct and indirect effects of a single nutrient pulse in a seaweed-epiphyte-graqer system. Mar Ecol Prog Ser 202:283–288
Zangerl AR and Berenbaum MR (1997) Cost of chemically defending seeds: furanocoumarins and Pastinaca sativa. Am Nat 150:491–504
Acknowledgements
We thank Nina Heikkilä, Anne Muola and Roosa Leimu for valuable laboratory and field assistance, and the Archipelago Research Institute of the University of Turku for providing facilities. We are grateful to Riitta Koivikko and Johanna Oja for conducting the chemical analyses. The insightful comments of the two anonymous referees are greatly appreciated. The research was funded by the Academy of Finland (Projects 44086 and 53832) and by the Wihuri Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christian Koerner
Rights and permissions
About this article
Cite this article
Honkanen, T., Jormalainen, V. Genotypic variation in tolerance and resistance to fouling in the brown alga Fucus vesiculosus . Oecologia 144, 196–205 (2005). https://doi.org/10.1007/s00442-005-0053-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0053-0