Abstract
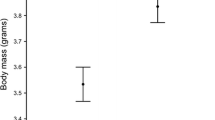
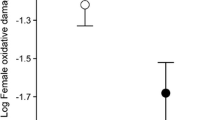
Carotenoids are antioxidant pigments involved in several physiological processes and signalling in animals that cannot synthesise them and therefore must acquire them from food. We experimentally investigated the effects of carotenoid availability in the diet during egg laying on antioxidant deposition in egg yolk and the related effects on nestling condition, female body condition and parental investment in the blue tit (Parus caeruleus). Carotenoid supplementation of egg-laying females resulted in a significant increase in carotenoid concentration in egg yolk, but not in vitamin E or A concentration. There was no relationship between yellow plumage colour of adult females and carotenoid deposition in eggs, and no differential effect of feeding treatment depending on female colour. Nestlings from eggs laid by carotenoid supplemented females had longer tarsi, had faster development of the immune system as reflected by leukocyte concentration in blood, and grew brighter yellow feathers than nestlings from control females. However, nestlings from the two groups did not differ significantly in body mass, plasma antioxidants or plumage colour hue. At the time of chick rearing, carotenoid-fed females had increased plasma vitamin E levels compared to controls. However, females from the two treatment groups did not differ significantly in body condition or feeding rate. These results suggest that carotenoid availability is limiting during egg laying, and that females may have to balance the benefits of investing in egg quality against the potential costs of impairing their own future antioxidant protection. In addition, there may be considerable variation in carotenoid availability not only across seasons, but also among different stages of the breeding season.



Similar content being viewed by others
References
Blount JD, Houston DC, Møller AP (2000) Why egg yolk is yellow. Trends Ecol Evol 15:47–49
Blount JD, Surai PF, Nager RG, Houston DC, Møller AP, Trewby ML, Kennedy MW (2002) Carotenoids and egg quality in the lesser black-backed gull (Larus fuscus): a supplemental feeding study of maternal effects. Proc R Soc Lond B 269:29–36
Blount JD, Houston DC, Surai PF, Møller AP (2004) Egg-laying capacity is limited by carotenoid pigment availability in wild gulls Larus fuscus. Proc R Soc Lond B 271(Suppl):S79–S81
Bortolotti GR, Negro JJ, Surai PF, Prieto P (2003) Carotenoids in eggs and plasma of red-legged partridges: effects of diet and reproductive output. Physiol Biochem Zool 76:367–374
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretic approach. Springer, Berlin Heidelburg New York
Burri BJ (2000) Carotenoids and gene expression. Nutrition 16:577–578
Chew BP (1993) Role of carotenoids in the immune response. J Dairy Sci 76:2804–2811
Edge R, McGarvey DJ, Truscott TG (1997) The carotenoids as anti-oxidants—a review. J Photochem Photobiol B 41:189–200
Endler JA (1990) On the measurement and classification of colour in studies of animal colour patterns. Biol J Linn Soc 41:315–352
Freckelton RP (2002) On the misuse of residuals in ecology: regression of residuals vs. multiple regression. J Anim Ecol 71:542–545
Goldstein H (2003) Multilevel statistical models, 3rd edn. Hodder Arnold, London
Goodwin TW (1984) The biochemistry of carotenoids. Chapman and Hall, London
Haq A, Bailey CA, Chinnah A (1996) Effect of β–carotene, canthaxanthin, lutein, and vitamin E on neonatal immunity of chicks when supplemented in the broiler breeder diets. Poult Sci 75:1092–1097
Hill GE, Inouye CY, Montgomerie R (2002) Dietary carotenoids predict plumage coloration in wild house finches. Proc R Soc Lond B 269:1119–1124
Hõrak P, Vellau H, Ots I, Møller AP (2000) Growth conditions affect carotenoid-based plumage coloration of great tit nestlings. Naturwissenschaften 87:460–464
Hõrak P, Surai PF, Møller AP (2002) Fat-soluble antioxidants in the eggs of great tits Parus major in relation to breeding habitat and laying sequence. Avian Sci 2:123–130
Inborr J (1996) Astaxantin—a carotenoid with great potential. Poult Int 35:54–60
Kemp C, Wylie L, Fisher C (2001) Broiler breeder nutrition, nutrient transfer and broiler performance. In: Proceedings of 13th European symposium on poultry nutrition. Blankenberge Belgium, pp 61–67
Koutsos EA, Clifford AJ, Calvert CC, Klasing KC (2003) Maternal carotenoid status modifies the incorporation of dietary carotenoids into immune tissues of growing chickens (Gallus gallus domesticus). J Nutr 133:1132–1138
Lessells CM, Boag PT (1987) Unrepeatable repetabilities: a common mistake. Auk 104:116–121
Lindén M, Møller AP (1989) Cost of reproduction and covariation of life history traits in birds. Trends Ecol Evol 4:367–371
Llaurado LI, Francesch A, Hernandez JM, Brafau J (1997) Effect of canthaxanthin supplementation on the hatchability of eggs of broiler breeders. In: Proceedings of 11th European symposium on poultry nutrition, Faaborg, Denmark, pp 280–282
Møller AP, Biard C, Blount JD, Houston DC, Ninni P, Saino N, Surai PF (2000) Carotenoid-dependant signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult Biol Rev 11:137–159
Møller AP, Erritzøe J, Saino N (2003) Seasonal changes in immune response and parasite impact on hosts. Am Nat 161:657–671
Monaghan P, Nager RG (1997) Why don’t birds lay more eggs? Trends Ecol Evol 12:270–274
Monaghan P, Nager RG, Houston DC (1998) The price of eggs: increased investment in egg production reduces the offspring rearing capacity of parents. Proc R Soc Lond B 265:1731–1735
Mousseau TA, Fox CW (eds) (1998) Maternal effects as adaptations. Oxford University Press, New York
Nilsson J-Å, Råberg L (2001) The resting metabolic cost of egg-laying and nestling feeding in great tits. Oecologia 128:187–192
van Noordwijk AJ, McCleery RH, Perrins CM (1995) Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J Anim Ecol 64:451–458
Nordling D, Andersson M, Zohari S, Gustafsson L (1998) Reproductive effort reduces specific immune response and parasite resistance. Proc R Soc Lond B 265:1291–1298
Olson VA, Owens IPF (1998) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13:510–514
Ots I, Murumägi A, Hõrak P (1998) Haematological health state indices of reproducing great tits: methodology and sources of natural variation. Funct Ecol 12:700–707
Partali V, Liaaen-Jensen S, Slagsvold T, Lifjeld JT (1987) Carotenoids in food chain studies. II The food chain of Parus spp. Monitored by carotenoid analysis. Comp Biochem Physiol B 87:885–888
Saino N, Stradi RD, Ninni P, Pini E, Møller AP (1999) Carotenoid plasma concentration, immune profile and plumage ornementation of male barn swallows (Hirunda rustica). Am Nat 154:441–448
Saino N, Ferrari RP, Romano M, Martinelli R, Møller AP (2003) Experimental manipulation of egg carotenoids affects immunity of barn swallow nestlings. Proc R Soc Lond B 270:2485–2489
Saks L, Ots I, Hõrak P (2003) Carotenoid-based plumage coloration of male greenfinches reflects health and immunocompetence. Oecologia 134:301–307
Salvante KG, Williams TD (2002) Vitellogenin dynamics during egg-laying: daily variation, repeatability and relationship with egg size. J Avian Biol 33:391–398
Slagsvold T, Lifjeld JT (1985) Variation in plumage colour of the great tit Parus major in relation to habitat, season and food. J Zool 206:321–328
Stearns SC (1989) Trade-offs in life history evolution. Funct Ecol 3:259–268
Sturkie PD (1986) Avian physiology, 4th edn. Springer, Berlin Heidelburg New York
Surai PF (2000) Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Brit Poult Sci 41:235–243
Surai PF (2002) Natural antioxidants in avian nutrition and reproduction. Nottingham University Press, Nottingham
Surai PF, Sparks NHC (2001) Comparative evaluation of the effect of two maternal diets on fatty acids, vitamin E and carotenoids in the chick embryo. Brit Poult Sci 42:252–259
Surai PF, Speake BK (1998) Distribution of carotenoids from the yolk to the tissues of the chick embryo. J Nutr Biochem 9:645–651
Surai PF, Ionov IA, Kuchmistova EF, Noble RC, Speake BK (1998) The relationship between the levels of α-tocopherol and carotenoids in the maternal feed, yolk and neonatal tissues: comparison between the chicken, turkey, duck and goose. J Sci Food Agric 76:593–598
Surai PF, Speake BK, Noble RC, Sparks NHC (1999) Tissue-specific antioxidant profiles and susceptibility to lipid peroxidation of the newly hatched chick. Biol Trace Elem Res 68:63–78
Surai PF, Speake BK, Sparks NHC (2001a) Carotenoids in avian nutrition and embryonic development 2 Antioxidant properties and discrimination in embryonic tissues. J Poult Sci 38:117–145
Surai PF, Speake BK, Wood NAR, Blount JD, Bortolotti GR, Sparks NHC (2001b) Carotenoid discrimination by the avian embryo: a lesson from wild birds. Comp Biochem Physiol B 128:743–750
Svensson E, Merilä J (1998) Molt and migratory condition in blue tits: a serological study. Condor 98:825–831
Visser ME, Lessells CM (2001) The costs of egg production and incubation in great tits (Parus major). Proc R Soc Lond B 268:1271–1277
Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM (1998) Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc R Soc Lond B 265:1867–1870
Zile MH (2001) Function of vitamin A in vertebrate embryonic development. J Nutr 131:705–708
Acknowledgements
Bird supplemental feeding, capture and ringing as well as egg and blood samplings were done under Ringing Licence (CRBPO Museum National d’Histoire Naturelle) and special authorisation for protected species (DIREN Champagne-Ardenne). Kemin Europa kindly provided samples of carotenoids (OroGlo Layer Dry). Fieldwork was done under permission of Parc Naturel Régional de la Forêt d’Orient and Office National des Forêts. We thank B. Auclair, S. Cassier and V. Coutouly for help in the field. J. Avilès, F. Cézilly, A. and V. Husté and three anonymous referees provided helpful comments on earlier version of the manuscript. J. M. Rossi developed software for reflectance spectrum analysis and colour parameter calculation. C.B. was supported by a doctoral grant from Ministère de l’Education et de la Recherche and P.F.S. was supported by the Scottish Executive Environment and Rural Affairs Department.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl
Rights and permissions
About this article
Cite this article
Biard, C., Surai, P.F. & Møller, A.P. Effects of carotenoid availability during laying on reproduction in the blue tit. Oecologia 144, 32–44 (2005). https://doi.org/10.1007/s00442-005-0048-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0048-x