Abstract
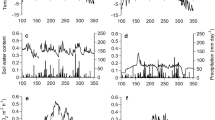
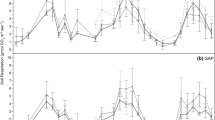
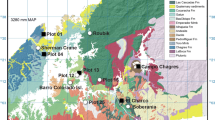
We measured the response of dark respiration (Rd) to temperature and foliage characteristics in the upper canopies of tree species in temperate rainforest communites in New Zealand along a soil chronosequence (six sites from 6 years to 120,000 years). The chronosequence provided a vegetation gradient characterised by significant changes in soil nutrition. This enabled us to examine the extent to which changes in dark respiration can be applied across forest biomes and the utility of scaling rules in whole-canopy carbon modelling. The response of respiration to temperature in the dominant tree species differed significantly between sites along the sequence. This involved changes in both Rd at a reference temperature (R10) and the extent to which Rd increased with temperature (described by Eo, a parameter related to the energy of activation, or the change in Rd over a 10°C range, Q10). Site averaged Eo ranged from 44.4 kJ mol−1 K−1 at the 60-year-old site to 26.0 kJ mol−1 K−1 at the oldest, most nutrient poor, site. Relationships between respiratory and foliage characteristics indicated that both the temperature response of respiration (Eo or Q10) and the instantaneous rate of respiration increased with both foliar nitrogen and phosphorus content. The ratio of photosynthetic capacity (Whitehead et al. in Oecologia 2005) to respiration (Amax/Rd) attained values in excess of 15 for species in the 6- to 120-year-old sites, but thereafter decreased significantly to around five at the 120,000-year-old site. This indicates that shoot carbon acquisition is regulated by nutrient limitations in the retrogressing ecosystems on the oldest sites. Our findings indicate that respiration and its temperature response will vary according to soil age and, therefore, to soil nutrient availability and the stage of forest development. Thus, variability in respiratory characteristics for canopies should be considered when using models to integrate respiration at large spatial scales.




Similar content being viewed by others
References
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Alward RD, Detling JK, Milchunas DG (1999) Grassland vegetation changes and nocturnal global warming. Science 283:229–231
Amthor JS (1989) Respiration and crop productivity. Springer, Berlin Heidelberg New York
Amthor JS (1997) Plant respiratory responses to elevated CO2 partial pressure. In: Allen LH, Kirkbaum MH, Olszyck DM, Whitman CE (eds) Advances in carbon dioxide effects research, ASA Special Publication No. 61. ASA/CSSA/SSSA, Madison
Amthor JS (2000a) Direct effect of elevated CO2 on nocturnal in situ leaf respiration in nine temperate deciduous tree species is small. Tree Physiol 20:139–144
Amthor JS (2000b) The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later. Ann Bot 86:1–20
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351
Atkin OK, Holly C, Ball MC (2000) Acclimation of snow gum (Eucalyptus pauciflora) leaf respiration to seasonal and diurnal variations in temperature: the importance of changes in the capacity and temperature sensitivity of respiration. Plant Cell Environ 23:15–26
Berry JA, Raison JK (1981) Responses of macrophytes to temperature. In: Lange OL, Nobel PS, Osmond CB, Zeigler H (eds) Physiological plant ecology I. responses to the physical environment. Springer, Berlin Heidelberg New York, pp 277–338
Bolstad PV, Mitchell K, Vose JM (1999) Foliar temperature-respiration response functions for broad- leaved tree species in the southern Appalachians. Tree Physiol 19:871–878
Cannell MGR, Thornley JHM (2000) Modelling the components of plant respiration: some guiding principles. Ann Bot 85:45–54
Dewar RC, Medlyn BE, McMurtrie RE (1999) Acclimation of the respiration/photosynthesis ratio to temperature: insights from a model. Glob Change Biol 5:615–622
Easterling DR et al. (1997) Maximum and minimum temperature trends for the globe. Science 277:364–367
Gifford RM (2003) Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol 30:171–186
Gonzalez-Meler MA, Giles L, Thomas RB, Siedow JN (2001) Metabolic regulation of leaf respiration and alternative pathway activity in response to phosphate supply. Plant Cell Environ 24:205–215
Griffin KL et al. (2002b) Leaf respiration is differentially affected by leaf vs. stand-level night-time warming. Glob Change Biol 8:479–485
Griffin KL, Tissue DT, Turnbull MH, Schuster W, Whitehead D (2001) Leaf dark respiration as a function of canopy position in Nothofagus fusca trees grown at ambient and elevated CO2 partial pressures for 5 years. Funct Ecol 15:497–505
Griffin KL, Turnbull M, Murthy R (2002) Canopy position affects the temperature response of leaf respiration in Populus deltoides. New Phytol 154:609–619
Gunderson CA, Norby RJ, Wullschleger SD (2000) Acclimation of photosynthesis and respiration to simulated climatic warming in northern and southern populations of Acer saccharum: laboratory and field evidence. Tree Physiol 20:87–96
Houghton RA (1993) The role of the world’s forests in global warming. In: Ramakrishna K, Woodwell GM (eds) World forest for the future: their use and conservation. Yale University Press, New Haven, pp 21–58
IPCC (2001) Third assessment report of Working Group I. United Nations Environmental Programme, Geneva Switzerland
Knorr W, Heimann M (2001) Uncertainties in global terrestrial biosphere modeling 1. A comprehensive sensitivity analysis with a new photosynthesis and energy balance scheme. Global Biogeochem Cy 15:207–225
Lambers H, Chapin III FS, Pons TL (1998) Plant physiological ecology. Springer, Berlin Heidelberg New York
Landsberg JJ, Waring RH (1997) A generalised model of forest productivity using simplified concepts of radiation-use efficiency, carbon balance and partitioning. Forest Ecol Manag 95:209–228
Larigauderie A, Körner C (1995) Acclimation of leaf dark respiration to temperature in alpine and lowland plant species. Ann Bot 76:245–252
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8:315–323
Loveys BR, Atkinson LJ, Sherlock DJ, Roberts RL, Fitter AH, Atkin OK (2003) Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Glob Change Biol 9: 895–910
Meir P, Grace J, Miranda AC (2001) Leaf respiration in two tropical rainforests: constraints on physiology by phosphorus, nitrogen and temperature. Funct Ecol 15:378–387
Mitchell KA, Bolstad PV, Vose JM (1999) Interspecific and environmentally induced variation in foliar dark respiration among eighteen southeastern deciduous tree species. Tree Physiol 19:861–870
Reich PB et al. (1998) Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: a test across biomes and functional groups. Oecologia 114:471–482
Reich PB, Oleksyn J, Tjoelker MG (1996) Needle respiration and nitrogen concentration in Scots Pine populations from a broad latitudinal range: a common garden test with field-grown trees. Funct Ecol 10:768–776
Richardson SJ, Peltzer D, Allen RB, McGlone M, Parfitt RL (2004) Rapid development of phosphorus limitation in temperate rainforest along the Franz Josef soil chronosequence. Oecologia 139:267–276
Ryan MG (1991a) Effects of climate change on plant respiration. Ecol Appl 1:157–167
Ryan MG (1991b) A simple method for for estimating gross carbon budgets for vegetation in forest ecosystems. Tree Physiol 1:255–266
Ryan MG (1995) Foliar maintenance respiration of subalpine and boreal trees and shrubs in relation to nitrogen content. Plant Cell Environ 18:765–772
Ryan MG, Linder S, Vose JM, Hubbard RM (1994) Dark respiration of pines. Ecol Bull 43:50–63
Ryan MG, Hubbard RM, Pongracic S, Raison RJ, McMurtrie R (1996) Foliage, fine-root, woody-tissue and stand respiration in Pinus radiata in relation to nitrogen status. Tree Physiol 16:333–343
Shenk HJ (1996) Modeling the effects of temperature on growth and persistence of tree species: A critical review of tree population models. Ecol Model 92:1–32
Shugart HH, Smith TM (1996) A review of forest patch models and their application to global change research. Climatic Change 34:131–153
Shugart HH, Smith TM, Post WM (1992) The potential for application of individual-based simulation models for assessing the effects of global change. Annu Rev Ecol Syst 23:15–38
Stevens PR (1968) A chronosequence of soils near Franz Josef Glacier. PhD Thesis, University of Canterbury, Christchurch
Stockfors J, Linder S (1998) The effect of nutrition on the seasonal course of needle respiration in Norway spruce stands. Trees 12:130–138
Thornley JHM, Cannell MGR (2000) Modelling the components of plant respiration: respresentation and realism. Ann Bot 85:55–67
Tissue DT, Lewis JD, Wullschleger SD, Amthor JS, Griffin KL, Anderson R (2002) Leaf respiration at different canopy positions in sweetgum (Liquidambar styraciflua) grown in ambient and elevated concentrations of carbon dioxide in the field. Tree Physiol 22:1157–1166
Tjoelker MG, Oleksyn J, Reich PB (1999) Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Glob Change Biol 5:679–691
Tjoelker MG, Oleksyn J, Reich PB (2001) Modelling respiration of vegetation: evidence for a general temperature-dependent Q(10). Glob Change Biol 7:223–230
Turnbull MH, Whitehead D, Tissue DT, Schuster W, Brown KJ, Griffin KL (2001) The response of leaf respiration to temperature and leaf characteristics in three deciduous tree species differs at sites of contrasting soil moisture. Tree Physiol 21:571–578
Turnbull MH, Whitehead D, Tissue DT, Schuster WSF, Brown KJ, Griffin KL (2003) Scaling foliar respiration in two contrasting forest canopies. Funct Ecol 17 :101–114
Valentini R et al. (2000) Respiration as the main determinant of carbon balance in European forests. Nature 404:861–865
Walker LR, del Moral R (2003) Primary succession and ecosystem rehabilitation. Cambridge University Press, Cambridge
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components. Princeton University Press, Princeton
Whitehead D et al. (2005) Photosynthesis and leaf reflectance indices for rainforest species in ecosystems undergoing progression and retrogression along a soil fertility chronosequence in New Zealand. Oecologia (in press)
Williams M, Eugster W, Rastetter EB, McFadden JP, Chapin FS (2000) The controls on net ecosystem productivity along an Arctic transect: a model comparison with flux measurements. Glob Change Biol 6:116–126
Acknowledgements
The authors gratefully acknowledge the New Zealand Foundation for Research, Science and Technology for providing the core funding that supports this research. KLG and DTT were supported by a National Science Foundation International Opportunities grant. We are indebted to the New Zealand Department of Conservation for providing access to the Westland field sites. The assistance of John Hunt, Adrian Walcroft and Margaret Barbour in sample collection is gratefully acknowledged. The experiments described here comply with the current laws of New Zealand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turnbull, M.H., Tissue, D.T., Griffin, K.L. et al. Respiration characteristics in temperate rainforest tree species differ along a long-term soil-development chronosequence. Oecologia 143, 271–279 (2005). https://doi.org/10.1007/s00442-004-1803-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1803-0