Abstract
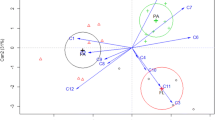
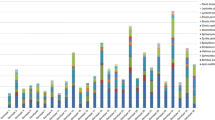
We investigated scent composition and pollinator attraction in two closely related orchids, Gymnadenia conopsea (L.) R.Br. s.l. and Gymnadenia odoratissima (L.) Rich. in four populations during the day and night. We collected pollinators of both species using hand nets and sampled floral odour by headspace sorption. We analysed the samples by gas chromatography with mass spectrometry to identify compounds and with electroantennographic detection to identify compounds with physiological activity in pollinators. In order to evaluate the attractiveness of the physiologically active compounds, we carried out trapping experiments in the field with single active odour substances and mixtures thereof. By collecting insects from flowers, we caught eight pollinators of G. conopsea, which were members of four Lepidoptera families, and 37 pollinators of G. odoratissima, from five Lepidopteran families. There was no overlap in pollinator species caught from the two orchids using nets. In the scent analyses, we identified 45 volatiles in G. conopsea of which three (benzyl acetate, eugenol, benzyl benzoate) were physiologically active. In G. odoratissima, 44 volatiles were identified, of which seven were physiologically active (benzaldehyde, phenylacetaldehyde, benzyl acetate, 1-phenyl-2,3-butandione, phenylethyl acetate, eugenol, and one unknown compound). In field bioassays using a mixture of the active G. odoratissima compounds and phenylacetaldehyde alone we caught a total of 25 moths, some of which carried Gymnadenia pollinia. A blend of the active G. conopsea volatiles placed in the G. odoratissima population did not attract any pollinators. The two orchids emitted different odour bouquets during the day and night, but G. odoratissima showed greater temporal differences in odour composition, with phenylacetaldehyde showing a significant increase during the night. The species differed considerably in floral odour emission and this differentiation was stronger in the active than non-active compounds. This differentiation of the two species, especially in the emission of active compounds, appears to have evolved under selection for attraction of different suites of Lepidopteran pollinators.




Similar content being viewed by others
References
Ågren L, Borg-Karlson AK (1984) Responses of Argogorytes (Hymenoptera: Sphecidae) males to odour signals from Ophrys insectifera (Orchidaceae). Preliminary EAG and chemical investigation. Nova Acta Regiae Soc Sci Ups V:111–117
Andersson S (2003a) Antennal responses to floral scents in the butterflies Inachis io, Aglais urticae (Nymphalidae), and Gonepterix rhamni (Pieridae). Chemoecology 13:13–20
Andersson S (2003b) Foraging responses in the butterflies Inachis io, Aglais urticae (Nymphalidae), and Gonepteryx rhamni (Pieridae) to floral scents. Chemoecology 13:1–11
Andersson S, Dobson HEM (2003) Antennal responses to floral scents in the butterfly Heliconius melpomene. J Chem Ecol 29:2319–2330
Andersson S, Nilsson LA, Groth I, Bergström G (2002) Floral scents in butterfly-pollinated plants: possible convergence in chemical composition. Bot J Linn Soc 140:129–153
Bateman RM, Hollingsworth PM, Preston J, Yi-Bo L, Pridgeon AM, Chase MW (2003) Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae). Bot J Linn Soc 142:1–40
Blight MM, LeMetayer M, Delegue MHP, Picket JA, Marion-Poll F, Wadhams LJ (1997) Identification of floral volatiles involved in recognition of oilseed rape flowers, Brassica napus by honeybees, Apis mellifera. J Chem Ecol 23:1715–1727
Brantjes NBM (1984) Unidirectional isolation in Gymnadenia spp. (Orchidaceae). Acta Bot Neerl 33:368
Cantello WW, Jakobson M (1979) Phenylacetaldehyde attracts moths to bladder flower and to blacklight traps. Environ Entomol 8:444–447
Creighton CS, McFadden TL, Cuthbert ER (1973) Supplementary data on phenylacetaldehyde: an attractant for Lepidoptera. J Econ Entomol 66:114–115
Daly KC, Smith BH (2000) Associative olfactory learning in the moth Manduca sexta. J Exp Biol 203:2025–2038
Daly KC, Durtschi ML, Smith BH (2001) Olfactory-based discrimination learning in the moth, Manduca sexta. J Insect Physiol 47:375–384
Darwin C (1862) On the various contrivances by which British and foreign orchids are fertilised by insects, and on the good effects of intercrossing. Murray, London
Dobson HEM (1994) Floral volatiles in insect biology. In: Bernays E (ed) Insect–plant interactions, vol 5. CRC, Boca Raton, Fla., pp 47–81
Dobson HEM, Danielson EM, Van Wesep ID (1999) Pollen odor chemicals as modulators of bumble bee foraging on Rosa rugosa Thunb. (Rosaceae). Plant Spec Biol 14:153–166
Dodson CH, Dressler RL, Hills HG, Adams RM,Williams NH (1969) Biologically active compounds in orchid fragrances. Science 164:1243–1249
Dudareva N, Pichersky E (2000) Biochemical and molecular genetic aspects of floral scents. Plant Physiol 122:627–633
Faegri K, van der Pijl L (1979) The principles of pollination ecology. Pergamon, Oxford
Fulton M, Hodges SA (1999) Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proc R Soc Lond B 266:2247–2252
Grant V (1949) Pollination systems as isolating mechanisms in angiosperms. Evolution 3:82–97
Grant V (1994) Modes and origins of mechanical and ethological isolation in angiosperms. Proc Natl Acad Sci U S A 91:3–10
Gregg KB (1983) Variation in floral fragrances and morphology: incipient speciation in Cycnoches? Bot Gaz 144:566–576
Haynes KF, Zhao JZ, Latif A (1991) Identification of floral compounds from Abelia grandiflora that stimulate upwind flight in cabbage looper moths. J Chem Ecol 17:637–646
Heath RR, Landolt PJ, Dueben B, Lenczewski B (1992) Identification of floral compounds of night-blooming jessamine attractive to cabbage looper moths. Environ Entomol 21:854–859
Hegi G (1980) Illustrierte Flora von Mitteleuropa, 3rd edn. Parey, Berlin
Hess HE, Landolt E, Hirzel R (1976) Flora der Schweiz und angrenzender Gebiete, vol 1. Birkhäuser, Basel, pp 620–622
Hodges SA (1997) Floral nectar spurs and diversification. Int J Plant Sci 158:81–88
Hodges SA, Whittall JB, Fulton M, Yang JY (2002) Genetics of floral traits influencing reproductive isolation between Aquilegia formosa and Aquilegia pubescens. Am Nat 159:S51-S60
Honda K, Ômura H, Hayashi N (1998) Identification of floral volatiles from Ligustrum japonicum that stimulate flower-visiting by cabbage butterfly, Pieris rapae. J Chem Ecol 24:2167–2180
Jakobsen HB, Olsen CE (1994) Influence of climatic factors on emission of flower volatiles in-situ. Planta 192:365–371
Johnson SD, Steiner KE (2000) Generalization versus specialization in plant pollination systems. Trends Ecol Evol 15:140–143
Jürgens A, Witt T, Gottsberger G (2003) Flower scent composition in Dianthus and Saponaria species (Caryophyllaceae) and its relevance for pollination biology and taxonomy. Biochem Syst Ecol 31:345–357
Kaiser R (1993) The scent of orchids. Elsevier, Amsterdam
Knudsen JT (2002) Variation in floral scent composition within and between populations of Genoma macrostachys (Arecaceae) in the western Amazon. Am J Bot 89:1772–1778
Knudsen JT, Tollsten L, Bergström LG (1993) Floral scents—a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33:253–280
Kullenberg B (1961) Studies in Ophrys pollination. Zool Bidr Uppsala. 34
Loughrin JH, Hamilton-Kemp TR, Andersen RA, Hildebrand DF (1990) Volatiles from flowers of Nicotiana sylvestris, N. otophora and Malus×domestica: Headspace components and day/night changes in their relative concentrations. Phytochemistry 29:2473–2477
Maadt J, Alexandersson R (2004) Variable selection in Platanthera bifolia (Orchidaceae): phenotypic selection differed between sex functions in a drought year. J Evol Biol 17:642–650
Matile P, Altenburger R (1988) Rhythms of fragrance emission in flowers. Planta 174:242–247
McKay JK, Lätta RG (2002) Adaptive population divergence: markers, Qtl and traits. Trends Ecol Evol 17:285–291
Menzel R (1985) Learning in honey bees in an ecological and behavioral context. In: Hölldobler B, Lindauer M (eds) Experimental behavioral ecology. Fischer, Stuttgart, pp 55–74
Möseler BM (1987) Zur morphologischen, phänologischen und standörtlichen Charakterisierung von Gymnadenia conopsea (L.) R. BR. ssp. densiflora. Florist Rundbr 21:8–21
Nilsson LA (1983) Processes of isolation and introgressive interplay between Platanthera bifolia (L.) Rich and P. chlorantha (Custer) Reichb. (Orchidaceae). Bot J Linn Soc 87:325–350
Omura H, Honda K, Hayashi N (2000) Floral scent of Osmanthus fragrans discourages foraging behavior of cabbage butterfly, Pieris rapae. J Chem Ecol 26:655–666
Pellmyr O (1986) Three pollination morphs in Cimicifuga simplex; incipient speciation due to inferiority competition. Oecologia 68:304–307
Plepys D, Ibarra F, Francke W, Löfstedt C (2002a) Odour-mediated nectar foraging in the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae): behavioural and electrophysiological responses to floral volatiles. Oikos 99:75–82
Plepys D, Ibarra F, Löfstedt C (2002b) Volatiles from flowers of Platanthera bifolia (Orchidaceae) attractive to the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae). Oikos 99:69–74
Raguso RA, Pichersky E (1995) Floral volatiles from Clarkia breweri and C. concinna (Onagraceae): recent evolution of floral scent and moth pollination. Plant Syst Evol 194:55–67
Raguso RA, Willis MA (2002) Synergy between visual and olfactory cues in nectar feeding by naive hawkmoths, Manduca sexta. Anim Behav 64:685–695
Raguso RA, Levin RA, Foose SE, Holmberg MW, Mcdade LA (2003) Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry 63:265–284
Reinhard HR, Gölz P, Peter R, Wildermuth H (1991) Die Orchideen der Schweiz und angrenzender Gebiete. Photorotar, Egg
Schiestl FP, Marion-Poll F (2002) Detection of physiologically active flower volatiles using gas chromatography coupled with electroantennography. In: Jackson JF, Linskens HF, Inman R (eds) Molecular methods of plant analysis, vol 21. Analysis of taste and aroma. Springer, Berlin Heidelberg New York, pp 173–198
Schiestl FP, Ayasse M, Paulus HF, Löfstedt C, Hansson BS, Ibarra F, Francke W (1999) Orchid pollination by sexual swindle. Nature 399:421–422
Schiestl FP, Peakall R, Mant J, Ibarra F, Schulz C, Francke S, Francke W (2003) The chemistry of sexual deception in an orchid-wasp pollination system. Science 302:437–438
Schomburg G (1990) Gas chromatography. A practical course. VCH, Weinheim
Soliva M, Widmer A (1999) Genetic and floral divergence among sympatric populations of Gymnadenia conopsea s.l. (Orchideaceae) with different flowering phenology. Int J Plant Sci 160:897–905
Stpiczynska M (2001) Osmophores of the fragrant orchid Gymnadenia conopsea L. (Orchidaceae). Acta Soc Bot Pol 70:91–96
Van der Cingel NA (1995) An atlas of orchid pollination. European orchids. Balkema, Rotterdam
Van der Pijl L, Dodson CH (1966) Orchid flowers: their pollination and evolution. University of Miami Press, Coral Gables, Fla.
Vöth W (2000) Gymnadenia, Nigritella und ihre Bestäuber. J Eur Orchid 32(3–4):547–573
Waser NM (1986) Flower constancy: definition, cause and measurement. Am Nat 127:593–603
Waser NM, Chittka L, Price MV, Williams NM, Ollerton J (1996) Generalization in pollination systems, and why it matters. Ecology 77:1043–1060
Acknowledgements
We would like to thank Caroline Weckerle (Zürich) and Charlotte Salzmann (Zürich) for assistance in the field and help on the manuscript and in the laboratory. Jim Mant (Zürich) provided helpful comments on the manuscript. We also thank Alex Kocyan (München) and Ruedi Irniger (Zürich) for information about Gymnadenia populations and Amots Dafni (Haifa) for discussion and helpful suggestions on this work. Financial support was provided by the ETH Zürich and the University of Zürich.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huber, F.K., Kaiser, R., Sauter, W. et al. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae). Oecologia 142, 564–575 (2005). https://doi.org/10.1007/s00442-004-1750-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1750-9