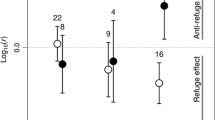
Abstract
We investigated the role of freshwater macrophytes as refuge by testing the hypothesis that predators capture fewer prey in more dense and structurally complex habitats. We also tested the hypothesis that habitat structure not only affects the prey-capture success of a single predator in isolation, but also the effectiveness of two predators combined, particularly if it mediates interactions between the predators. We conducted a fully crossed four-factorial laboratory experiment using artificial plants to determine the separate quantitative (density) and qualitative (shape) components of macrophyte structure on the prey-capture success of a predatory damselfly, Ischnura heterosticta tasmanica, and the southern pygmy perch, Nannoperca australis. Contrary to our expectations, macrophyte density had no effect on the prey-capture success of either predator, but both predators were significantly less effective in the structurally complex Myriophyllum analogue than in the structurally simpler Triglochin and Eleocharis analogues. Furthermore, the greater structural complexity of Myriophyllum amplified the impact of the negative interaction between the predators on prey numbers; the habitat use by damselfly larvae in response to the presence of southern pygmy perch meant they captured less prey in Myriophyllum. These results demonstrate habitat structure can influence multiple predator effects, and support the mechanism of increased prey refuge in more structurally complex macrophytes.

Similar content being viewed by others
References
Beck MW (2000) Separating the elements of habitat structure: independent effects of habitat complexity and structural components on rocky intertidal gastropods. J Exp Mar Biol Ecol 249:29–49
Beckerman AP, Uriarte M, Schmitz OJ (1997) Experimental evidence for a behaviour-mediated trophic cascade in a terrestrial food chain. Proc Natl Acad Sci U S A 94:10735–10738
Bettoli PW, Maceina MJ, Noble RL, Betsill RK (1992) Piscivory in largemouth bass as a function of aquatic vegetation abundance. N Am J Fish Manage 12:509–516
Carlisle DM, Hawkins CP (1998) Relationships between invertebrate assemblage structure, two trout species, and habitat structure in Utah mountain lakes. J N Am Benthol Soc 17:286–300
Chilton EWI (1990) Macroinvertebrate communities associated with three aquatic macrophytes (Ceratophyllum demersum, Myriophyllum spicatum,and Vallisneria americana) in Lake Onalaska, Wisconsin. J Freshwater Ecol 5:455–466
Coen LD, Heck KLJ, Abele LG (1981) Experiments on competition and predation among shrimps of seagrass meadows. Ecology 62:1484–1893
Coull BC, Wells JBJ (1983) Refuges from fish predation: experiments with phytal meiofauna from the New Zealand rocky intertidal. Ecology 64:1599–1609
Crowder LB, Cooper WE (1982) Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63:1802–1813
Crowder LB, Squires DB, Rice JA (1997) Nonadditive effects of terrestrial and aquatic predators on juvenile estuarine fish. Ecology 78:1796–1804
Crowder LB, McCollum EW, Martin TH (1998) Changing perspectives on food web interactions in lake littoral zones. In: Jeppesen E, Sondergaard M, Sondergaard M, Christoffersen K (eds) The structuring roles of submerged macrophytes in lakes. Springer, Berlin Heidelberg New York, pp 240–249
Cyr H, Downing JA (1988) The abundance of phytophilous invertebrates on different species of submerged macrophytes. Freshwater Biol 20:365–374
Davies PE, Humphries P (1996) An environmental flow study of the Meander, Macquarie and South Esk Rivers, Tasmania. Department of Primary Industry and Fisheries
Dean RL, Connell JH (1987) Marine invertebrates in an algal succession. III. Mechanisms linking habitat complexity with diversity. J Exp Mar Biol Ecol 109:249–273
Dettmers JM, Wahl DH, Soluk DA, Gutreuter S (2001) Life in the fast lane: fish and foodweb structure in the main channel of large rivers. J N Am Benthol Soc 20:255–265
Diehl S (1988) Foraging efficiency of three freshwater fishes: effects of structural complexity and light. Oikos 53:207–214
Diehl S, Kornijow R (1998) Influence of submerged macrophytes on trophic interactions among fish and macroinvertebrates. In: Jeppesen E, Sondergaard M, Sondergaard M, Christoffersen K (eds) The structuring roles of submerged Macrophytes in lakes. Springer, Berlin Heidelberg New York, pp 24–46
Dionne M, Folt CL (1991) An experimental analysis of macrophyte growth forms as fish foraging habitat. Can J Fish Aquat Sci 48:123–131
Dionne M, Butler M, Folt C (1990) Plant-specific expression of anti-predator behaviour by larval damselflies. Oecologia 83:371–377
Edgar GJ (1983) The ecology of south-east Tasmanian phytal animal communities. I. Spatial organization on a local scale. J Exp Mar Biol Ecol 70:129–157
Folsom TC, Collins NC (1984) The diet and foraging behaviour of the larval dragonfly Anax junius (Aeshnidae), with an assessment of the role of refuges and prey activity. Oikos 42:105–113
Gilinsky E (1984) The role of fish predation and spatial heterogeneity in determining benthic community structure. Ecology 65:455–468
Gotceitas V, Colgan P (1989) Predator foraging success and habitat complexity: quantitative test of the threshold hypothesis. Oecologia 80:158–166
Heads PA (1985) The effect of invertebrate and vertebrate predators on the foraging movements of Ischnura elegans larvae (Odonata: Zygoptera). Freshwater Biol 15:559–571
Heck KLJ, Crowder LB (1991) Habitat structure and predator–prey interactions in vegetated aquatic systems. In: Bell SS, McCoy ED, Mushinsky HR (eds) Habitat structure: the physical arrangement of objects in space. Chapman and Hall, London, pp 281–299
Heck KLJ, Orth RJ (1980) Seagrass habitats: the roles of habitat complexity, competition and predation in structuring associated fish and motile macroinvertebrate assemblages. In: Kennedy VS (ed) Estuarine perspectives. Academic Press, London, pp 449–464
Heck KLJ, Thoman TA (1981) Experiments on predator–prey interactions in vegetated aquatic habitats. J Exp Mar Biol Ecol 53:125–134
Heck KLJ, Wetstone GS (1977) Habitat complexity and invertebrate species richness and abundance in tropical seagrass meadows. J Biogeogr 4:135–142
Humphries P (1995) Life history, food and habitat of southern pygmy perch, Nannoperca australis, in the Macquarie River, Tasmania. Mar Freshwater Res 46:1159–11
Humphries P (1996) Aquatic macrophytes, macroinvertebrate associations and water levels in a lowland Tasmanian river. Hydrobiologia 321:219–233
Humphries P, Davies PE, Mulcahy ME (1996) Macroinvertebrate assemblages of littoral habitats in the Macquarie and Mersey Rivers, Tasmania: implications for the management of regulated rivers. Regul River 12:99–122
Jacobsen L, Perrow MR, Landkildehus F, Hjorne M, Lauridsen TL, Berg S (1997) Interactions between piscivores, zooplanktivores and zooplankton in submerged macrophytes: preliminary observations from enclosure and pond experiments. Hydrobiologia 342/343:197–205
James PL, Heck KLJ (1994) The effects of habitat complexity and light intensity on ambush predation within a simulated seagrass habitat. J Exp Mar Biol Ecol 176:187–200
Koperski P (1997) Changes in feeding behaviour of the larvae of the damselfly Enallagma cyathigerum in response to stimuli from predators. Ecol Entomol 22:167–175
Leber KM (1985) The influence of predatory decapods, refuge, and microhabitat selection on seagrass communities. Ecology 66:1951–1964
Lipcius RN, Eggleston DB, Miller DL, Luhrs TC (1998) The habitat–survival function for Caribbean spiny lobster: an inverted size effect and non-linearity in mixed algal and seagrass habitats. Mar Freshwater Res 49:807–816
Losey JE, Denno RF (1988) Positive predator–predator interactions: enhanced predation rates and synergistic suppression of aphid populations. Ecology 79:2143–2152
Main KL (1987) Predator avoidance in seagrass meadows: prey behaviour, microhabitat selection, and cryptic coloration. Ecology 68:170–180
Martin TH, Wright RA, Crowder LB (1989) Non-additive impact of blue crabs and spot on their prey assemblages. Ecology 70:1935–1942
McCoy ED, Bell SS (1991) Habitat structure: the evolution and diversification of a complex topic. In: McCoy ED, Bell SS, Mushinsky HR (eds) Habitat structure: the physical arrangement of objects in space. Chapman and Hall, London, pp 3–27
McIntosh AR, Peckarsky BL (1996) Differential behavioural responses of mayflies from streams with and without fish to trout odour. Freshwater Biol 35:141–148
McPeek MA, Peckarsky BL (1998) Life histories and the strengths of species interactions: combining mortality, growth, and fecundity effects. Ecology 79:867–879
Morin PJ (1995) Functional redundancy, non-additive interactions, and supply-side dynamics in experimental pond communities. Ecology 76:133–149
Nelson WG (1979) Experimental studies of selective predation on amphipods: consequences for amphipod distribution and abundance. J Exp Mar Biol Ecol 38:225–245
Nelson WG, Bonsdorff E (1990) Fish predation and habitat complexity: are complexity thresholds real? J Exp Mar Biol Ecol 141:183–194
Orr BK, Resh VH (1991) Interactions among aquatic vegetation, predators and mosquitoes: implications for management of Anopheles mosquitoes in a freshwater marsh. Proc Calif Mosq Vector Control Assoc 58:214–220
Orr BK, Resh VH (1992) Influence of Myriophyllum aquaticum cover on Anopheles mosquito abundance, oviposition and larval microhabitat. Oecologia 90:474–482
Persson L, Eklov P (1995) Prey refuges affecting interactions between piscivorous perch and juvenile perch and roach. Ecology 76:70–81
Pierce CL (1988) Predator avoidance, microhabitat shift, and risk-sensitive foraging in larval dragonflies. Oecologia 77:81–90
Polis GA (1989) Exploitation competition and the evolution of interference, cannabalism, and intraguild predation in age/size structured populations. In: Ebenman B, Persson L (eds) Size-structured populations. Springer, Berlin Heidelberg New York, pp 185–202
Power ME (1992) Habitat heterogeneity and the functional significance of fish in river food webs. Ecology 73:1675–1688
Rooke B (1986) Macroinvertebrates associated with macrophytes and plastic imitations in the Eramosa River, Ontario, Canada. Arch Hydrobiol 106:307–325
Ryer C (1988) Pipefish foraging: effects of fish size, prey size and altered habitat complexity. Mar Ecol Prog Ser 48:37–45
Savino JF, Stein RA (1982) Predator–prey interaction between largemouth bass and bluegills as influenced by simulated, submersed vegetation. Trans Am Fish Soc 111:255–266
Savino JF, Stein RA (1989) Behavioural interactions between fish predators and their prey: effects of plant density. Anim Behav 37:311–321
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355
Soluk DA (1993) Multiple predator effects: predicting combined functional response of stream fish and invertebrate predators. Ecology 74:219–225
Soluk DA, Collins NC (1988) Synergistic interactions between fish and stoneflies: facilitation and interference among stream predators. Oikos 52:94–100
Soluk DA, Richardson JS (1997) The role of stoneflies in enhancing growth of trout: a test of the importance of predator–predator facilitation within a stream community. Oikos 80:214–219
Stoner AW (1982) The influence of benthic invertebrates on the foraging behaviour of pinfish, Lagodon rhomboides (Linnaeus). J Exp Mar Biol Ecol 58:271–284
Stoner AW, Lewis FGI (1985) The influence of quantitative and qualitative aspects of habitat complexity in tropical seagrass meadows. J Exp Mar Biol Ecol 94:49–40
Swisher BJ, Soluk DA, Wahl DH (1998) Non-additive predation in littoral habitats: influences of habitat complexity. Oikos 81:30–37
Walsh EJ (1995) Habitat-specific predation susceptibilities of a littoral rotifer to two invertebrate predators. Hydrobiologia 313/314:205–211
Warfe DM (2003) The role of habitat structure in a freshwater food web. PhD dissertation. University of Tasmania
Werner EE, Gilliam JF, Hall DJ, Mittelbach GG (1983) An experimental test of the effects of predation risk on habitat use in fish. Ecology 64:1540–1548
Wilkinson L (1999) SYSTAT, version 9. SYSTAT, Evanston, Ill.
Acknowledgments
We are grateful to Mr Henry Foster of Fosterville for access to the Macquarie River on his property. Mr Glenn Macpherson and Dr David Ratkowsky, both of the University of Tasmania, provided statistical advice during the planning of this investigation. The Inland Fisheries Service of Tasmania and the Animal Ethics Committee of the University of Tasmania provided the relevant permits and approvals to conduct this research. We would also like to thank Dr Todd Crowl (University of Utah), Dr Dan Soluk (University of Illinois), and two anonymous referees who provided useful comments on an earlier version of this manuscript, while William Elvey and Brett Mawbey provided valuable logistical support at various stages of the investigation. D. M. W. was supported by an Australian Postgraduate Award, and part of L. A. B.’s contribution was supported by a University of Tasmania Study Leave grant while at the University of Canterbury, Christchurch, New Zealand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warfe, D.M., Barmuta, L.A. Habitat structural complexity mediates the foraging success of multiple predator species. Oecologia 141, 171–178 (2004). https://doi.org/10.1007/s00442-004-1644-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1644-x