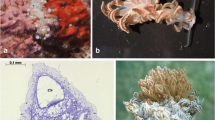
Abstract
Larvae of the sessile marine invertebrate Bugula neritina (Bryozoa) are protected by an effective chemical defense. From the larvae, we isolated three bryostatin-class macrocyclic polyketides, including the novel bryostatin 20, that deterred feeding by a common planktivorous fish that co-occurs with B. neritina. A unique bacterial symbiont of B. neritina, Endobugula sertula, was hypothesized as the putative source of the bryostatins. We show that: (1) bryostatins are concentrated in B. neritina larvae and protect them against predation by fish; (2) the adults are not defended by bryostatins; and (3) E. sertula produces bryostatins. This study represents the first example from the marine environment of a microbial symbiont producing an anti-predator defense for its host and, in this case, specifically for the host’s larval stage, which is exceptionally vulnerable to predators.






Similar content being viewed by others
References
Adams SM (1976) Feeding ecology of eelgrass fish communities. Trans Am Fish Soc 105:514–519
Anthoni U, Nielsen PH, Pereira M, Christophersen C (1990) Bryozoan secondary metabolites: a chemotaxonomical challenge. Comp Biochem Physiol B 96:431–437
Ayling AM (1981) The role of biological disturbance in temperate subtidal encrusting communities. Ecology 62:830–847
Bullard SG, Hay ME (2002) Palatability of marine macro-holoplankton: Nematocysts, nutritional quality, and chemistry as defenses against consumers. Limnol Oceanogr 47:1456–1467
Cheplick GP, Clay K (1988) Acquired chemical defences in grasses: the role of fungal endophytes. Oikos 52:309–318
Clay K (1988) Fungal endophytes of grasses: a defensive mutualism between plants and fungi. Ecology 69:10–16
Clay K (2001) Symbiosis and the regulation of communities. Am Zool 41:810–824
Davidson SK, Haygood MG (1999) Identification of sibling species of the bryozoan Bugula neritina that produce different anticancer bryostatins and harbor distinct strains of the bacterial symbiont Candidatus Endobugula sertula. Biol Bull 196:273–280
Davidson SK, Allen SW, Lim GE, Anderson CM, Haygood MG (2001) Evidence for the biosynthesis of bryostatins by the bacterial symbiont Candidatus Endobugula sertula of the bryozoan Bugula neritina. Appl Environ Microbiol 67:4531–4537
Davis AR (1988) Colony regeneration following damage and size-dependent mortality in the Australian ascidian Podoclavella moluccensis (Sluiter). J Exp Mar Biol Ecol 123:269–286
Elyakov GB, Kuznetsova T, Mikhailov VV, Maltsev II, Voinov VG, Fedoreyev SA (1991) Brominated diphenyl ethers from a marine bacterium associated with the sponge Dysidea sp. Experientia 47:632–633
Faulkner DJ, Harper MK, Salomon CE, Schmidt EW (1999) Localisation of bioactive metabolites in marine sponges. Mem Queensl Museum 44:167–173
Feeny PP (1991) The evoloution of chemical ecology: contributions from the study of herbivorous insects. In: Rosenthal GA, Berenbaum M (eds) Herbivores: their interactions with secondary plant metabolites, vol 2. Academic Press, San Diego, Calif., pp 1–35
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Gil-Turnes MS, Fenical W (1992) Embryos of Homarus americanus are protected by epibiotic bacteria. Biol Bull 182:105–108
Gil-Turnes MS, Hay ME, Fenical W (1989) Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 246:116–118
Harvell CD, West JM, Griggs C (1996) Chemical defense of embryos and larvae of a West Indian gorgonian coral, Briareum asbestinum. Invertebr Reprod Dev 30:239–246
Hay ME (1996) Marine chemical ecology: what’s known and what’s next? J Exp Mar Biol Ecol 200:103–134
Haygood MG, Davidson SK (1997) Small-subunit rRNA genes and in situ hybridization with oligonucleotides specific for the bacterial symbionts in the larvae of the bryozoan Bugula neritina and proposal of “Candidatus Endobugula sertula”. Appl Environ Microbiol 63:4612–4616
Haygood MG, Davidson SK (1998) Bacterial symbionts of the bryostatin-producing bryozoan Bugula neritina. In: LeGal Y, Halvorson HO (eds) New developments in marine biotechnology. Plenum Press, New York, pp 281–284
Haygood MG, Schmidt EW, Davidson SK, Faulkner DJ (1999) Microbial symbionts of marine invertebrates: opportunities for microbial biotechnology. J Mol Microbiol Biotechnol 1:33–43
Heck KL, Pennock JR, Valentine JF, Coen LD, Sklenar SA (2000) Effects of nutrient enrichment and small predator density on seagrass ecosystems: an experimental assessment. Limnol Oceanogr 45:1041–1057
Iyengar EV, Harvell CD (2001) Predator deterrence of early developmental stages of temperate lecithotrophic asteroids and holothuroids. J Exp Mar Biol Ecol 264:171–188
Jackson JBC (1977) Competition on marine hard substrata: the adaptive significance of solitary and colonial strategies. Am Nat 111:743–768
Jackson JBC (1985) Distribution and ecology of clonal and aclonal benthic invertebrates. In: Jackson JBC, Buss LW, Cook RE (eds) Population biology and evolution of clonal organisms. Yale University Press, New Haven, Conn., pp 297–355
Kellner RLL (2001) Suppression of pederin biosynthesis through antibiotic elimination of endosymbionts in Paederus sabaeus. J Insect Physiol 47:475–483
Kellner RLL (2002) Molecular identification of an endosymbiotic bacterium associated with pederin biosynthesis in Paederus sabaeus (Coleoptera: Staphylinidae). Insect Biochem Mol Biol 32:389–395
Kellner RLL, Dettner K (1995) Allocation of pederin during lifetime of Paederus rove beetles (Coleoptera: Staphylinidae): evidence for polymorphism of hemolymph toxin. J Chem Ecol 21:1719–1733
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144
Kobayashi J, Ishibashi M (1993) Bioactive metabolites of symbiotic marine microorganisms. Chem Rev 93:1753–1769
Kott P, Parry DL, Cox GC (1984) Prokaryotic symbionts with a range of ascidian hosts. Bull Mar Sci 34:308–312
Lindquist N (1996) Palatability of invertebrate larvae to corals and sea anemones. Mar Biol 126:745–755
Lindquist N (2002) Chemical defense of early life stages of benthic marine invertebrates. J Chem Ecol 28:1987–2000
Lindquist N, Fenical W (1991) New tambjamine class alkaloids from the marine ascidian Atapozoa sp. and its nudibranch predators. Origin of the tambjamines in Atapozoa. Experientia 47:504–506
Lindquist N, Hay ME (1995) Can small rare prey be chemically defended? The case for marine larvae. Ecology 76:1347–1358
Lindquist N, Hay ME (1996) Palatability and chemical defense of marine invertebrate larvae. Ecol Monogr 66:431–450
Lindquist N, Hay ME, Fenical W (1992) Defense of ascidians and their conspicuous larvae: adult vs larval chemical defenses. Ecol Monogr 62:547–568
Lopanik NB (2003) Ontogenetic production of a chemical defense in Bugula neritina (Bryozoa): chemistry, quantification, symbiont-mediated biosynthesis and gene expression. In: Marine studies. University of Delaware, Newark, Del., p 145
Lucas JS, Hart RJ, Howden ME, Salathe R (1979) Saponins in eggs and larvae of Acanthaster planci (L.) (Asteroidea) as chemical defences against planktivorous fish. J Exp Mar Biol Ecol 40:155–165
Madigan MT, Martinko JM, Parker J (1997) Brock biology of microorganisms, 8th edn. Prentice-Hall, Upper Saddle River, N.J.
McClintock JB, Baker BJ (1997) Palatability and chemical defense of eggs, embryos and larvae of shallow-water Antarctic marine invertebrates. Mar Ecol Prog Ser 154:121–131
McClintock JB, Baker BJ (eds) (2001) Marine chemical ecology. CRC Press, New York
McGovern TM, Hellberg ME (2003) Cryptic species, cryptic endosymbionts, and geographical variation in chemical defences in the bryozoan Bugula neritina. Mol Ecol 12:1207–1215
Mendola D (2000) Aquacultural production of bryostatin 1 and ecteinascidin 743. In: Fusetani N (ed) Drugs from the sea. Karger, Basel, Switzerland pp 120–133
Morrison TB, Weis JJ, Wittwer CT (1998) Quantification of low-copy transcripts by continuous SYBR (R) green I monitoring during amplification. Biotechniques 24:954–959
Muyzer G, Dewaal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis: analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Nelson WG (1979) Experimental studies of selective predation on amphipods: consequences for amphipod distribution and abundance. J Exp Mar Biol Ecol 38:225–245
Olson RR (1985) The consequences of short-distance larval dispersal in a sessile marine invertebrate. Ecology 66:30–39
Pawlik JR (1993) Marine invertebrate chemical defenses. Chem Rev 93:1911–1922
Pettit GR (1996) Progress in the discovery of biosynthetic anticancer drugs. J Nat Prod 59:812–821
Pettit GR, Day JF, Hartwell JL, Wood HB (1970) Antineoplastic components of marine animals. Nature 227:962–963
Poecke RMP van, Dicke M (2002) Induced parasitoid attraction by Arabidopsis thaliana: involvement of the octadecanoid and the salicylic acid pathway. J Exp Bot 53:1793–1799
Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS (2002) A cross-ecosystem comparison of the strength of trophic cascades. Ecol Lett 5:785–791
Sipura M (1999) Tritrophic interactions: willows, herbivorous insects and insectivorous birds. Oecologia 121:537–545
Sokal RR, Rohlf FJ (1995) Biometry. Freeman, New York
Stierle AC, Cardellina II JH, Singleton FL (1988) A marine Micrococcus produces metabolites ascribed to the sponge Tedania ignis. Experientia 44:1021
Vacelet J, Donadey C (1977) Electron microscope study of the association between some sponges and bacteria. J Exp Mar Biol Ecol 30:301–314
Walters LJ (1992) Post-settlement success of the arborescent bryozoan Bugula neritina (L.): the importance of structural complexity. J Exp Mar Biol Ecol 164:55–71
Watson JD (1976) Molecular biology of the gene. Benjamin, Menlo Park, Calif.
Wilkinson CR, Nowak M, Austin B, Colwell RR (1981) Specificity of bacterial symbionts in Mediterranean and Great Barrier Reef sponges. Microb Ecol 7:13–21
Woollacott RM (1981) Association of bacteria with bryozoan larvae. Mar Biol 65:155–158
Woollacott RM, Zimmer RL (1975) A simplified placenta-like system for the transport of extraembryonic nutrients during embryogenesis of Bugula neritina (Bryozoa). J Morphol 147:355–378
Young CM (1986) Direct observations of field swimming behavior in larvae of the colonial ascidian Ecteinascidia turbinata. Bull Mar Sci 39:279–289
Young CM, Bingham BL (1987) Chemical defense and aposematic coloration in larvae of the ascidian Ecteinascidia turbinata. Mar Biol 96:539–544
Young CM, Chia FS (1987) Abundance and distribution of pelagic larvae as influenced by predation, behavior and hydrographic factors. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates, vol 9. General aspects: seeking unity in diversity. Blackwell, Palo Alto, Calif., pp 385–463
Acknowledgements
We would like to thank Barb Campbell, Kathy Coyne, Lisa Waidner, and Jon McLaughlin for their help with the molecular portions of this project. We are grateful for collection support provided by Channing Jones, Jeremy Weisz, and Glenn Safrit. Sigma Xi Grants in Aid of Research to N.B.L. and SG project R/F-9 to N.M.T funded portions of this project. We would like to thank two anonymous reviewers for comments that substantially improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Lopanik, N., Lindquist, N. & Targett, N. Potent cytotoxins produced by a microbial symbiont protect host larvae from predation. Oecologia 139, 131–139 (2004). https://doi.org/10.1007/s00442-004-1487-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1487-5