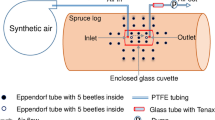
Abstract
Bark beetles engage in one of the most pronounced examples of group procurement of defended plants. Their aggregation pheromones attract both sexes and are essential to overcome constitutive and rapidly inducible lethal defenses. The relative benefits to senders versus receivers of these signals are only partly understood. Because the initial stage of host entry can be hazardous, there may be benefit to a cheating strategy, whose practitioners respond to pheromones but do not engage in host searching. Several disadvantages to cheating have been proposed, but the role of predators has not been considered. Predators exploit bark beetle pheromones to locate prey, accumulate at the breeding site, and consume adult bark beetles before they enter the tree. Preliminary experiments quantified arrival patterns in the field. We used a laboratory assay to investigate relative predation on pioneers (those that initially select and enter hosts) and responders (those that arrive at a host in response to pheromones) during host colonization. Our model system utilized the pine engraver, Ips pini, which exhibits male harem polygamy. We allowed male I. pini to colonize host tissue and added females 1 day later. Also 1 day later, we variably added additional males and predacious checkered beetles, Thanasimus dubius. These treatments included two densities of males and three densities of predators that were selected to emulate field conditions. Responding males experienced higher predation than pioneers. T. dubius ate more males than females, independent of the presence or absence of responding males. T. dubius affected the distribution of females per male, although the number of females that survived to construct ovipositional galleries was constant. We discuss the viability of cheating, implications for biological control, and predator-prey coevolution in this cooperative, group-colonizing herbivore.


Similar content being viewed by others
References
Anderson RF (1948) Host selection by the pine engraver. J Econ Entomol 41:596–602
Aukema BH, Raffa KF (2000) Chemically mediated predator-free space: herbivores can synergize intraspecific communication without increasing risk of predation. J Chem Ecol 26:1923–1939
Aukema BH, Raffa KF (2002) Relative effects of exophytic predation, endophytic predation, and intraspecific competition on a subcortical herbivore: consequences to the reproduction of Ips pini and Thanasimus dubius. Oecologia 133:483–491
Aukema BH, Dahlsten DL, Raffa KF (2000) Improved population monitoring of bark beetles and predators by incorporating disparate behavioral responses to semiochemicals. Environ Entomol 29:618–629
Aviles L (2002) Solving the freeloaders paradox: genetic associations and frequency-dependent selection in the evolution of cooperation among nonrelatives. Proc Natl Acad Sci USA 99:14268–14273
Berryman AA, Dennis B, Raffa KF, Stenseth NC (1985) Evolution of optimal group attack, with particular reference to bark beetles (Coleoptera: Scolytidae). Ecology 66:898–903
Birgersson G, Schlyter F, Bergstrom G, Lofqvist J (1988) Individual variation in aggregation pheromone content of the bark beetle, Ips typographus. J Chem Ecol 14:1737–1761
Bunt WD, Coster JE, Johnson PC (1980) Behavior of the southern pine beetle on the bark of host trees during mass attack. Ann Entomol Soc Am 73:647–652
Byers JA (1999) Effects of attraction radius and flight paths on catch of scolytid beetles dispersing outward through rings of pheromone traps. J Chem Ecol 25:985–1005
Candolin U (1998) Reproduction under predation risk and the trade-off between current and future reproduction in the threespine stickleback. Proc R Soc London Ser B 265:1171–1175
Christiansen E, Waring RH, Berryman AA (1987) Resistance of conifers to bark beetle attack—searching for general relationships. For Ecol Manage 22:89–106
Codella SG, Raffa KF (1995) Contributions of female oviposition patterns and larval behavior to group defense in conifer sawflies (Hymenoptera, Diprionidae). Oecologia 103:24–33
Copeland J, Moiseff A (1995) The occurrence of synchrony in the North American firefly Photinus carolinus (Coleoptera, Lampyridae). J Insect Behav 8:381–394
Coulson RN (1979) Population dynamics of bark beetles. Annu Rev Entomol 24:417–447
Cronin JT, Reeve JD, Wilkens R, Turchin P (2000) The pattern and range of movement of a checkered beetle predator relative to its bark beetle prey. Oikos 90:127–138
Erbilgin N, Raffa KF (2000) Effects of host tree species on attractiveness of tunneling pine engravers, Ips pini , to conspecifics and insect predators. J Chem Ecol 26:823–840
Erbilgin N, Raffa KF (2001) Modulation of predator attraction to pheromones of two prey species by stereochemistry of plant volatiles. Oecologia 127:444–453
Erbilgin N, Nordheim EV, Aukema BH, Raffa KF (2002) Population dynamics of Ips pini and Ips grandicollis in red pine plantations in Wisconsin: Within- and between-year associations with predators, competitors, and habitat quality. Environ Entomol 31:1043–1051
Frazier JL, Nebeker TE, Mizell RF, Calvert WH (1981) Predatory behavior of the clerid beetle Thanasimus dubius (Coleoptera: Cleridae) on the southern pine beetle (Coleoptera Scolytidae). Can Entomol 113:35–43
Gries G, Pierce HD, Lindgren BS, Borden JH (1988) New techniques for capturing and analyzing semiochemicals for scolytid beetles (Coleoptera: Scolytidae). J Econ Entomol 81:1715–1720
Harari AR, Ben-Yakir D, Rosen D (2000) Male pioneering as a mating strategy: the case of the beetle Maladera matrida. Ecol Entomol 25:387–394
Hedrick AV (2000) Crickets with extravagant mating songs compensate for predation risk with extra caution. Proc R Soc London Ser B 267:671–675
Hedrick AV, Dill LM (1993) Mate choice by female crickets is influenced by predation risk. Anim Behav 46:193–196
Herms DA, Haack RA, Ayres BD (1991) Variation in semiochemical-mediated prey-predator interaction: Ips pini (Scolytidae) and Thanasimus dubius (Cleridae). J Chem Ecol 17:515–524
Ihaka I, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314
Izhaki I, Maitav A (1998) Blackcaps Sylvia atricapilla stopping over at the desert edge: inter- and intrasexual differences in spring and autumn migration. Ibis 140:234–249
Kirkendall LR (1983) The evolution of mating systems in bark and ambrosia beetles (Coleoptera: Scolytidae and Platypodidae). Zool J Linn Soc 77:293–352
Klepzig KD, Smalley EB, Raffa KF (1996) Combined chemical defenses against an insect-fungal complex. J Chem Ecol 22:1367–1388
Kotiaho J, Alatalo RV, Mappes J, Parri S, Rivero A (1998) Male mating success and risk of predation in a wolf spider: a balance between sexual and natural selection? J Anim Ecol 67:287–291
Landolt PJ (1997) Sex attractant and aggregation pheromones of male phytophagous insects. Am Entomol 43:12–22
Michener G (1983) Spring emergence schedules and vernal behavior of Richardson’s ground squirrels: why do males emerge from hibernation before females? Behav Ecol Sociobiol 14:29–38
Miller DR, Gibson KE, Raffa KF, Seybold SJ, Teale SA, Wood DL (1997) Geographic variation in response of pine engraver, Ips pini , and associated species to pheromone, lanierone. J Chem Ecol 23:2013–2031
Moiseff A, Copeland J (2000) A new type of synchronized flashing in a North American firefly. J Insect Behav 13:597–612
Paynter QE, Anderbrant O, Schlyter F (1990) Behavior of male and female spruce bark beetles, Ips typographus , on the bark of host trees during mass attack. J Insect Behav 3:529–543
Polis GA, Barnes JD, Seely MK, Henschel JR, Enders MM (1998) Predation as a major cost of reproduction in Namib Desert tenebrionid beetles. Ecology 79:2560–2566
Pope DN, Coulson RN, Fargo WS, Gagne JA, Kelley CW (1980) The allocation process and between-tree survival probabilities in Dendroctonus frontalis infestations. Res Popul Ecol 22:197–210
Raffa KF (2001) Mixed messages across multiple trophic levels: the ecology of bark beetle chemical communication systems. Chemoecology 11:49–65
Raffa KF, Berryman AA (1983) The role of host plant resistance in the colonization behavior and ecology of bark beetles (Coleoptera: Scolytidae). Ecol Monogr 53:27–49
Raffa KF, Dahlsten DL (1995) Differential responses among natural enemies and prey to bark beetle pheromones. Oecologia 102:17–23
Raffa KF, Klepzig KD (1989) Chiral escape of bark beetles from predators responding to a bark beetle pheromone. Oecologia 80:566–569
Reeve JD (1997) Predation and bark beetle dynamics. Oecologia 112:48–54
Reynolds JD (1993) Should attractive individuals court more—theory and a test. Am Nat 141:914–927
Schlyter F, Anderbrant O (1993) Competition and niche separation between two bark beetles—existence and mechanisms. Oikos 68:437–447
Schlyter F, Birgersson G (1989) Individual variation of pheromone in bark beetles and moths—a comparison and an evolutionary background. Holarct Ecol 12:457–465
Schlyter F, Svensson M, Zhang QH, Knizek M, Krokene P, Ivarsson P, Birgersson G (2001) A model for peak and width of signaling windows: Ips duplicatus and Chilo partellus pheromone component proportions—does response have a wider window than production? J Chem Ecol 27:1481–1511
Seybold SJ, Ohtsuka T, Wood DL, Kubo I (1995) Enantiomeric composition of ipsdienol: a chemotaxonomic character for North American populations of Ips spp. in the pini subgeneric group (Coleoptera: Scolytidae). J Chem Ecol 21:995–1016
Sih A, Wooster DE (1994) Prey behavior, prey dispersal, and predator impacts on stream prey. Ecology 75:1199–1207
Slagsvold T, Dale S, Kruszewicz A (1995) Predation favors cryptic coloration in breeding male pied flycatchers. Anim Behav 50:1109–1121
Stephen FM, Dahlsten DL (1976) The arrival sequence of the arthropod complex following attack by Dendroctonus brevicomis (Coleoptera: Scolytidae) in ponderosa pine: the temporal and spatial arrival pattern of Dendroctonus brevicomis in ponderosa pine. Can Entomol 108:283–304
Stoddard PK (1999) Predation enhances complexity in the evolution of electric fish signals. Nature 400:254–256
Strom BL, Goyer RA, Shea PJ (2001) Visual and olfactory disruption of orientation by the western pine beetle to attractant-baited traps. Entomol Exp Appl 100:63–67
Thatcher RC, Pickard LS (1966) The clerid beetle, Thanasimus dubius , as a predator of the southern pine beetle. J Econ Entomol 59:955–957
Thomas JB (1961) The life history of Ips pini (Say) (Coleoptera: Scolytidae). Can Entomol 93:384–390
Turchin P, Taylor AD, Reeve JD (1999) Dynamical role of predators in population cycles of a forest insect: an experimental test. Science 285:1068–1070
Wagner TL, Fargo WS, Flamm RO, Coulson RN, Pulley PE (1987) Development and mortality of Ips calligraphus (Coleoptera: Scolytidae) at constant temperatures. Environ Entomol 16:484–496
Wallin KF, Raffa KF (2000) Influences of host chemicals and internal physiology on the multiple steps of postlanding host acceptance behavior of Ips pini (Coleoptera: Scolytidae). Environ Entomol 29:442–453
Wallin KF, Raffa KF (2002) Prior encounters modulate subsequent choices in host acceptance behavior by the bark beetle Ips pini. Entomol Exp Appl 103:205–218
Weslien J (1994) Interactions within and between species at different densities of the bark beetle Ips typographus and its predator Thanasimus formicarius. Entomol Exp Appl 71:133–143
Wood D (1982) The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu Rev Entomol 27:411–446
Zuk M, Kolluru GR (1998) Exploitation of sexual signals by predators and parasitoids. Q Rev Biol 73:415–438
Acknowledgements
S. LaFontaine, L. Kuiper, N. Erbilgin, E. Hladilek, J. Gruber, B. Burwitz, A. Boyd, and K. Gardner assisted in these assays. M. Clayton provided statistical advice. We thank the Wisconsin Department of Natural Resources for providing the trees and trapping sites. This study was supported by USDA NRI AMD 96 04317, the University of Wisconsin-Madison College of Agricultural and Life Sciences, S.C. Johnson and Son, Inc., and an Elsa and Louis Thomsen Wisconsin Distinguished Fellowship awarded to B.H.A. We thank D. Mahr, D. Hogg, A. Ives, R. Lindroth, J. Handelsman, D. Wood, J. Cronin, and two anonymous reviewers for their helpful discussions and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aukema, B.H., Raffa, K.F. Gender- and sequence-dependent predation within group colonizers of defended plants: a constraint on cheating among bark beetles?. Oecologia 138, 253–258 (2004). https://doi.org/10.1007/s00442-003-1433-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1433-y