Abstract
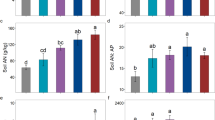
The effects of the Mediterranean shrub Cistus albidus on N cycling were studied in two siliceous (granitic-derived and schistic-derived) and one calcareous soil differentiated by their texture and acidity. We aimed to find out whether soils under C. albidus were affected by the release of C compounds from the canopy, and whether phenolic compound production in C. albidus changed depending on the soil N availability. Calcareous soils, with higher clay content and polyvalent cations, had a higher organic matter content but lower net N mineralization rates than siliceous soils, and C. albidus growing therein were characterized by lower foliar N and phenolic compound concentrations. Under C. albidus, all types of soils had higher phenolic compound concentrations and polyphenol oxidase activity. C. albidus presence and leachate addition decreased net N mineralization and increased soil respiration in siliceous soils, and these changes were related to a higher soil C/N ratio under the canopy. In calcareous soils, however, no significant effects of plant presence on N cycling were found. In the studied plant-soil system it is not likely that higher phenolic compound concentrations were selected during evolution to enhance nutrient conservation in soil because (1) higher phenolic compound concentrations were not associated with lower soil fertilities, (2) C compounds released from C. albidus accelerated N cycling by increasing N immobilization and no evidence was found for decreased gross N mineralization, and (3) soil organic N content was more related to soil chemical and physical properties than to the effects of the C. albidus canopy.





Similar content being viewed by others
References
Aguilera LE, Gutiérrez JR, Meserve PL (1999) Variation in soil micro-organisms and nutrients underneath and outside the canopy of Adesmia bedwellii (Papilionaceae) shrubs in arid coastal Chile following drought and above average of rainfall. J Arid Environ 42:61–70
Appel HM (1993) Phenolics in ecological interactions: the importance of oxidation. J Chem Ecol 19:1521–1552
Bending GD, Read DJ (1995) The structure and function of the vegetative mycelium of ectomycorrhizal plants. VI. Activities of nutrient mobilizing enzymes in birch litter colonized by Paxillus involutus (Fr.) Fr. New Phytol 130:411–417
Blum U (1998) Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J Chem Ecol 24:685–708
Blum U, Shafer SR (1988) Microbial populations and phenolic acids in soil. Soil Biol Biochem 20:793–800
Boufalis A, Pellissier F (1994) Allelopathic effects of phenolic mixtures on respiration of two spruce mycorrhizal fungi. J Chem Ecol 20:2283–2289
Bryant JP, Chapin FSI, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368
Claus H, Filip Z (1990) Effects of clays and other solids on the activity of phenoloxidases produced by some fungi and actinomycetes. Soil Biol Biochem 22:483–488
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230:895–899
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Feeny PP (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Fierer N, Schimel JP, Cates RG, Zou J (2001) Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol Biochem 33:1827–1839
Gershenzon J (1984) Changes in the levels of plant secondary metabolites under water and nutrient stress. Rec Adv Phytochem 18:273–320
Glyphis JP, Puttick GM (1989) Phenolics, nutrition and insect herbivory in some garrigue and maquis plant species. Oecologia 78:259–263
Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR (2001) The carbon-nutrient balance hypothesis: its rise and fall. Ecol Lett 4:86–95
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15:238–243
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Hook PB, Burke IC, Lauenroth WK (1991) Heterogenity of soil and plant N and C associated with individual plants and openings in North American shortgrass steppe. Plant Soil 138:247–256
Horner JD, Gosz JR, Cates RG (1988) The role of carbon-based plant secondary metabolites in decomposition in terrestrial ecosystems. Am Nat 132:869–883
Inderjit, Mallik AU (1999) Nutrient status of black spruce (Picea mariana [mill.] BSP) forest soils dominated by Kalmia angustifolia L. Acta Oecol 20:87–92
Koricheva J, Larsson S, Haukioja E, Keinänen M (1998) Regulation of woody plant secondary metabolism by resource availability: hypothesis testing by means of meta-analysis. Oikos 83:212–226
Kuiters AT (1990) Role of phenolic substances from decomposing forest litter in plant-soil interactions. Acta Bot Neerl 39:329–348
Laukkanen H, Julkunen-Tiitto R, Hohtola A (1997) Effect of different nitrogen nutrients on the viability, protein synthesis and tannin production of Scot pine callus. Physiol Plant 100:982–988
Leake JR, Read DJ (1990) Proteinase activity in mycorrhizal fungi. I. The effect of extracellular pH on the production and activity of proteinase by ericoid endophytes from soils of contrasted pH. New Phytol 115:243–250
Marigo G (1973) Sur une méthode de fractionnement et d'estimation des composés phénoliques chez les végétaux. Analusis 2:106–110
Mastrantonio M (2000) Master Thesis. Universitat Autònoma de Barcelona, Barcelona
Muller RN, Kalisz PJ, Kimmerer TW (1987) Intraspecific variation in production of astringent phenolics over a vegetation-resource availability gradient. Oecologia 72:211–215
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods in soil analysis. II. Chemical and microbiological properties. Soil Science Society of America, Madison, Wis., USA, pp 539–580
Nichols-Orians CM (1991) Condensed tannins, attine ants and the performance of a symbiotic fungus. J Chem Ecol 17:1177–1185
Nicolai V (1988) Phenolic and mineral content of leaves influences decomposition in European forest ecosystems. Oecologia 75:575–579
Northup RR, Dahlgren RA, Yu Z (1995a) Intraspecific variation of conifer phenolic concentration on a marine terrace soil acidity gradient; a new interpretation. Plant Soil 171:255–262
Northup RR, Yu Z, Dahlgren RA, Vogt KA (1995b) Polyphenol control of nitrogen release from pine litter. Nature 377:227–229
Northup RR, Dahlgren RA, McColl JG (1998) Polyphenols as regulators of plant-litter-soil interactions in northern California's pygmy forest: a positive feedback? Biogeochemistry 42:189–220
Oades JM (1988) The retention of organic matter in soils. Biogeochemistry 5:35–70
Palm CA, Sanchez PA (1990) Decomposition and nutrient release patterns of the leaves of three tropical legumes. Biotropica 22:330–338
Pind A, Freeman C, Lock MA (1994) Enzymic degradation of phenolic materials in peatlands- measurement of phenol oxidase activity. Plant Soil 159:227–231
Rice EL (1984) Allelopathy, 2nd edn. Academic Press, Orlando
Schimel JP, van Cleve K, Cates RG, Clausen TP, Reichardt PB (1996) Effects of balsam poplar (Populus balsamifera) tannins and low molecular weight phenolics on microbial activity in taiga floodplain soil: implications for changes in N cycling during succession. Can J Bot 74:84–90
Schmidt IK, Michelsen A, Jonasson S (1997) Effects of labile soil carbon on nutrient partitioning between an artic graminoid and microbes. Oecologia 112:557–565
Shafer SR, Blum U (1991) Influence of phenolic acids on microbial populations in the rhizosphere of cucumber. J Chem Ecol 17:369–388
Sparling GP, Ord BG, Vaughan D (1981) Changes in microbial biomass and activity in soils amended with phenolic acids. Soil Biol Biochem 13:455–460
Sugai SF, Schimel JP (1993) Decomposition and biomass incorporation of 14C-labeled glucose and phenolics in taiga forest-floor: effect of substrate quality, successional state, and season. Soil Biol Biochem 25:1379–1389
Tan KH (1996) Soil sampling, preparations and analysis. Dekker, New York, USA
Tang C, Cai W, Kohl K, Nishimoto RK (1995) Plant stress and allelopathy. In: Inderjit, Dakshini KMM, Einhellig FA (eds) Allelopathy: organisms, processes and applications. American Chemical Society, Washington, D.C., USA, pp 142–157
Vinton AM, Burke IC (1995) Interactions between individual plant species and soil nutrient status in shortgrass steppe. Ecology 76:1116–1133
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. Blackwell, Oxford
Whitehead DC, Dibb H, Hartley RD (1982) Phenolic compounds in soil as influenced by the growth of different plant species. J Appl Ecol 19:579–588
Zackrisson O, Nilsson MC (1992) Allelopathic effects by Empetrum hermaphroditum on seed germination of two boreal tree species. Can J For Res 22:1310–1319
Acknowledgements
The authors would like to thank Marta Mastrantonio, Jordi Martínez, Roger Puig, Silvia Artesero, Bernat Castells and Francesc Cebrià for field assistance. We also thank Josep Maria Alcañiz, Ramon Vallejo and David W. Valentine for critically reading this manuscript, and Oriol Ortiz for his expertise help in soil analyses. E.C. had a predoctoral fellowship (FPI) from the Ministerio de Educacion y Cultura (Spain) in collaboration with Carburos Metalicos SA. We are thankful for financial support from CICYT grants REN-2001–0003 and REN-2001–0278 (Spanish Government).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castells, E., Peñuelas, J. Is there a feedback between N availability in siliceous and calcareous soils and Cistus albidus leaf chemical composition?. Oecologia 136, 183–192 (2003). https://doi.org/10.1007/s00442-003-1258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1258-8