Abstract
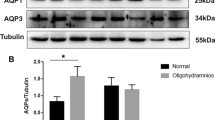
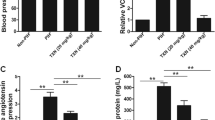
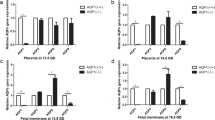
The mechanism of idiopathic oligohydramnios is still uncertain, and there is no effective and targeted treatment for it. Placental aquaporins (AQPs) were associated with idiopathic oligohydramnios. This study aimed to investigate the effect of tanshinone IIA on amniotic fluid volume (AFV) and its underlying molecular mechanisms related to placental AQPs (AQP1, AQP3, AQP8, AQP9). Results showed that compared with the women with normal AFV, placental AQP1, AQP3, AQP8, and AQP9 protein expressions were decreased in women with idiopathic oligohydramnios. Immunohistochemistry revealed localization of AQP1, AQP3, AQP8, and AQP9 mainly in trophoblast cells within labyrinth zone of mouse placenta. Also, AQP1 was located in fetal vascular endothelial cells. Pregnant mice were administered with tanshinone IIA (10 mg/kg or 50 mg/kg, n = 8, respectively) or vehicle (n = 8) from 9.5 to 18.5 gestational day (GD). Tanshinone IIA markedly increased the AFV in pregnant mice, without the effects on embryo numbers per litter, atrophic embryo rate, fetal weight, and placental weight, as well as increased the expressions of AQPs and inhibited the activity of GSK-3β in mice placenta. In JEG-3 cells, tanshinone IIA downregulated AQP1, AQP3, AQP8, AQP9 expressions and inhibited the activity of GSK-3β. Activating GSK-3β with MK-2206 eliminated these alterations. Thus, tanshinone IIA could increase AFV in pregnant mice, possibly through downregulating placental AQP1, AQP3, AQP8, and AQP9 expression via inhibiting the activity of GSK-3β. Tanshinone IIA may be optional for the treatment of idiopathic oligohydramnios.








Similar content being viewed by others
References
Beall MH, van den Wijngaard JP, van Gemert MJ, Ross MG (2007a) Regulation of amniotic fluid volume. Placenta 28:824–832
Beall MH, Wang S, Yang B, Chaudhri N, Amidi F, Ross MG (2007b) Placental and membrane aquaporin water channels: correlation with amniotic fluid volume and composition. Placenta 28:421–428
Chu H, Shen M (2008) Treating oligohydramnios with extract of Salvia miltiorrhiza: a randomized control trial. Ther Clin Risk Manag 4:287–290
Damiano A, Zotta E, Goldstein J, Reisin I, Ibarra C (2001) Water channel proteins AQP3 and AQP9 are present in syncytiotrophoblast of human term placenta. Placenta 22:776–781
Damiano AE, Zotta E, Ibarra C (2006) Functional and molecular expression of AQP9 channel and UT-A transporter in normal and preeclamptic human placentas. Placenta 27:1073–1081
Hua Y, Ding S, Cheng H, Luo H, Zhu X (2017) Tanshinone IIA increases aquaporins expression in human amniotic epithelial WISH cells by stimulating GSK-3beta phosphorylation. Clin Chim Acta 473:204–212
Huang Y, Long X, Tang J, Li X, Zhang X, Luo C, Zhou Y, Zhang P (2020) The attenuation of traumatic brain injury via inhibition of oxidative stress and apoptosis by tanshinone IIA. Oxid Med Cell Longev 2020:4170156
Jiang SS, Zhu XJ, Ding SD, Wang JJ, Jiang LL, Jiang WX, Zhu XQ (2012) Expression and localization of aquaporins 8 and 9 in term placenta with oligohydramnios. Reprod Sci 19:1276–1284
Kordowitzki P, Kranc W, Bryl R, Kempisty B, Skowronska A, Skowronski MT (2020) The relevance of aquaporins for the physiology, pathology, and aging of the female reproductive system in mammals. Cells 9(12)
Lin J, Song T, Li C, Mao W (2020) GSK-3beta in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim Biophys Acta Mol Cell Res 1867:118659
Lin L, Jadoon SS, Liu SZ, Zhang RY, Li F, Zhang MY, Ai-Hua T, You QY, Wang P (2019) Tanshinone IIA ameliorates spatial learning and memory deficits by inhibiting the activity of ERK and GSK-3beta. J Geriatr Psychiatry Neurol 32:152–163
Luo H, Liu Y, Song Y, Hua Y, Zhu X (2020) Aquaporin 1 affects pregnancy outcome and regulates aquaporin 8 and 9 expressions in the placenta. Cell Tissue Res 381:543–554
Mann SE, Ricke EA, Torres EA, Taylor RN (2005) A novel model of polyhydramnios: amniotic fluid volume is increased in aquaporin 1 knockout mice. Am J Obstet Gynecol 192:2041–4; discussion 4–6
Marino GI, Castro-Parodi M, Dietrich V, Damiano AE (2010) High levels of human chorionic gonadotropin (hCG) correlate with increased aquaporin-9 (AQP9) expression in explants from human preeclamptic placenta. Reprod Sci 17:444–453
Martinez N, Damiano AE (2017) Aquaporins in fetal development. Adv Exp Med Biol 969:199–212
Ozgen G, Dincgez Cakmak B, Ozgen L, Uguz S, Sager H (2022) The role of oligohydramnios and fetal growth restriction in adverse pregnancy outcomes in preeclamptic patients. Ginekol Pol 93: 3235–241
Perez-Perez A, Vilarino-Garcia T, Dietrich V, Guadix P, Duenas JL, Varone CL, Damiano AE, Sanchez-Margalet V (2020) Aquaporins and placenta. Vitam Horm 112:311–326
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256:385–387
Sha XY, Xiong ZF, Liu HS, Di XD, Ma TH (2011a) Maternal-fetal fluid balance and aquaporins: from molecule to physiology. Acta Pharmacol Sin 32:716–720
Sha XY, Xiong ZF, Liu HS, Zheng Z, Ma TH (2011b) Pregnant phenotype in aquaporin 8-deficient mice. Acta Pharmacol Sin 32:840–844
Shao H, Gao S, Ying X, Zhu X, Hua Y (2021) Expression and regulation of aquaporins in pregnancy complications and reproductive dysfunctions. DNA Cell Biol 40:116–125
Takahashi-Yanaga F, Sasaguri T (2009) Drug development targeting the glycogen synthase kinase-3beta (GSK-3beta)-mediated signal transduction pathway: inhibitors of the Wnt/beta-catenin signaling pathway as novel anticancer drugs. J Pharmacol Sci 109:179–183
Wang C, Li H, Zhou K, Luo C, Li Y, Xie L, Hua Y (2014) Sodium tanshinone IIA sulfonate and sodium danshensu open the placental barrier through down-regulation of placental P-glycoprotein in mice: implications in the transplacental digoxin treatment for fetal heart failure. Int J Cardiol 176:1331–1333
Wang H, Wu D, Cai L, Li X, Zhang Z, Chen S (2020) Aberrant methylation of WD-repeat protein 41 contributes to tumour progression in triple-negative breast cancer. J Cell Mol Med 24:6869–6882
Wang S, Chen J, Beall M, Zhou W, Ross MG (2004) Expression of aquaporin 9 in human chorioamniotic membranes and placenta. Am J Obstet Gynecol 191:2160–2167
Wang S, Kallichanda N, Song W, Ramirez BA, Ross MG (2001) Expression of aquaporin-8 in human placenta and chorioamniotic membranes: evidence of molecular mechanism for intramembranous amniotic fluid resorption. Am J Obstet Gynecol 185:1226–1231
Zhang Y, Can R, Mao C, Zhang Y, Zhu L, Shao H, Wang L, Wang A, Xu Z (2009) The effect of tanshinone IIA on renal and liver functions in ovine fetuses in utero. Drug Chem Toxicol 32:362–371
Zheng Z, Liu H, Beall M, Ma T, Hao R, Ross MG (2014) Role of aquaporin 1 in fetal fluid homeostasis. J Matern Fetal Neonatal Med 27:505–510
Zhu X, Jiang S, Hu Y, Zheng X, Zou S, Wang Y, Zhu X (2010) The expression of aquaporin 8 and aquaporin 9 in fetal membranes and placenta in term pregnancies complicated by idiopathic polyhydramnios. Early Hum Dev 86:657–663
Zhu XQ, Jiang SS, Zhu XJ, Zou SW, Wang YH, Hu YC (2009) Expression of aquaporin 1 and aquaporin 3 in fetal membranes and placenta in human term pregnancies with oligohydramnios. Placenta 30:670–676
Acknowledgements
We would like to thank all the authors’ work and the financial support by grants from the Project of Zhejiang Province Medical and Health Science, Technology Plan and the Science and Technology Bureau of Wenzhou and the Obstetrics and gynecology of combine traditional Chinese and Western medicine of Zhejiang Province. The study sponsors were not involved in the study collection, analysis and interpretation of data, or the writing of the article.
Funding
The work was supported by the Project of Zhejiang Province Medical and Health Science and Technology Plan (2021KY215), the Science and Technology Bureau of Wenzhou (Y20210026) and the Obstetrics and gynecology of combine traditional Chinese and Western medicine of Zhejiang Province (2017-XK-A42).
Author information
Authors and Affiliations
Contributions
HL. S. and SJ. P. contributed equally to this work. HL.S. designed and performed the experiments, drafted the article; SJ. P. performed the experiments and revised the article; YH. L. and XJ. C. collected the clinical specimens and analyzed the data; DR. D. and LL.P. analyzed the data; Y. H. revised the article critically for important intellectual content and final approval of the version to be published.
Corresponding author
Ethics declarations
Research involving human participants and animals
The research involving human was performed in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University (No.2016–28). The Animal experiment obtained ethics approval from the Laboratory Animal Ethics Committee of Wenzhou Medical University (No. wydw2019-0260).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shao, H., Pan, S., Lan, Y. et al. Tanshinone IIA increased amniotic fluid volume through down-regulating placental AQPs expression via inhibiting the activity of GSK-3β. Cell Tissue Res 389, 547–558 (2022). https://doi.org/10.1007/s00441-022-03646-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03646-5